3. Complement Fixation Test
The complement fixation test (CFT) was extensively used in syphilis serology after being introduced by Wasserman in 1909. It took a number of decades before the CFT was adapted for routine use in virology. CFT meet the following criteria; it is convenient and rapid to perform, the demand on equipment and reagents is small, and a large variety of test antigens are readily available. However, there is now a trend to replace the CFT with more direct, sensitive and rapid techniques, such as RIAs and EIAs. Although CFT is considered to be a relatively simple test, it is a very exacting procedure because 5 variables are involved. In essence the test consists of two antigen-antibody reactions, one of which is the indicator system. The first reaction, between a known virus antigen and a specific antibody takes place in the presence of a predetermined amount of complement. The complement is removed or "fixed" by the antigen-antibody complex. The second antigen-antibody reaction consists of reacting sheep rbc with haemolysin. When this indicator system is added to the reactants, the sensitized rbcs will only lyse in the presence of free complement. The antigens used for CFT tend to be group antigens rather than type-specific antigens. In order for the CFT to be set up correctly, the optimal concentration of haemolytic serum, complement, and antigen should be determined by titration. The following is a protocol for setting a complement fixation test.
a. Titration of haemolytic serum and complement
Dilutions of complement with 20% difference in concentration are made from 1:30 to 1:279. The following dilutions of haemolytic serum are made: 1:400, 1:800, 1:1600, 1:2000, 1:2400, 1:2800, 1:3200.
The following controls are required:
The optimal sensitizing concentration (OSC) of haemolytic serum is the dilution which gives the most lysis with the highest dilution of complement. One haemolytic dose of complement (HD50) is the dilution that gives 50% lysis at the OSC of haemolytic serum. 3 HD50 of complement is used for the CFT
b. Titration of antigen and antibody
Antigen at dilutions of 1:2 to 1:512 is titrated against positive serum control. The following controls are incorporated:
The optimal dose of the antigen is the highest dilution of antigen that gives 75% or more fixation with the highest dilution of antibody.
c. CFT proper
In the CF proper, the haemolytic serum is used at the optimal sensitizing concentration, the complement at 3HD50, and each individual antigen at the optimal dose. Patients' sera should be inactivated at 56oC for 30 minutes before. Screening is usually carried out at around 1:10 in VBS, if positive, the serum is retested with doubling dilutions of 1:10 to 1:80. Control plates should be prepared with all the antigens used included. The following controls should be present;-
All controls should show complete lysis and in the complement back-titration, the reading should be 0 at the second well and 1 to 2 at the third well. The highest dilution of patient serum that still shows a reading of 3 or 4 is the CF titre. Diagnosis of a recent infection is usually made by the detection of a fourfold or greater increase in titre or by the detection of a high antibody titre from a single specimen (1:80 or above). Where antigens used are grown from yolf sac, the sera should be tested against control yolk sac antigen in order to exclude the possibility of non-specific reaction.

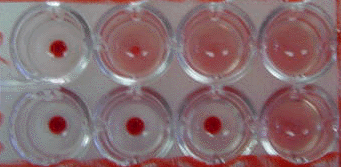
Complement Fixation Test in Microtiter Plate. Rows 1 and
2exhibit complement fixation obtained with acute and convalescent
phase serum specimens, respectively. (2-fold serum dilutions were
used) The observed 4-fold increase is significant and
indicates infection.
Advantages of CFT
Disadvantages of CFT