7. Immunofluorescense
Immunofluorescence (IF) is widely used for the rapid diagnosis of virus infections by the detection of virus antigen in clinical specimens, as well as the detection of virus-specific IgG or IgA or IgM antibody. The technique makes use of a fluorescein- labelled antibody to stain specimens containing specific virus antigens, so that the stained cells fluoresces under UV illumination. In the case of direct IF, the specimen is probed directly with a specific labelled antibody against a particular virus antigen. In the case of indirect IF, the specimen is first probed with a non-labelled specific antibody, followed by a labelled antibody against the first antibody. Direct or indirect IF can be used for the detection of virus antigen, whereas indirect IF is virtually always used for the detection of antibody. Indirect IF possess the advantage of an extra amplification step for the signal, however, it requires an extra step in comparison to direct IF.
Detection of viral antigens
IF is most commonly used for the detection of respiratory viruses in respiratory specimens. Nasopharyngeal aspirates are the best specimens to use and is usually collected from babies less than 12 months old. However, there are no reasons why nasopharyngeal aspirates cannot be collected from older children and adults. A number of respiratory viruses can be detected by direct or indirect IF, including RSV, influenza A and B, adenoviruses and parainfluenza viruses. However, the sensitivities vary greatly between different viruses. The method is most useful in the case of RSV where antiviral treatment is available for severely ill babies. IF is also widely used for the detection of HSV infections, from vesicle lesions and brain lesions, for VZV and CMV infections. However in the case of CMV infection, the sensitivity of IF on clinical specimens directly is low, with the possible exception of the CMV antigenaemia test.
A typical indirect IF procedure for the detection of viral antigens is as follows;- cells from the clinical specimen are immobilized onto individual wells on a slide. Specific polyclonal or monoclonal sera is then added to each well and the slide is incubated at 37oC for 30 to 60 minutes. The slide is then washed 3 times for 5 minutes each with PBS and fluorescein labelled antibody against the first antibody is added. The slide is further incubated at 37oC for 30 to 60 minutes and washed again. The slide is then prepared for microscopy. Specific monoclonal or polyclonal sera raised against the viral antigen can be used. Monoclonal sera offer the advantage of increased sensitivity and specificity. However, one must be certain that it can detect all the different strains of the virus.
IF is highly dependent on the quality of the specimen. In many instances it has proved to be more sensitive than equivalent EIAs. This is because a firm diagnosis can be made on the identification of a few cells only that contain fluorescence of the right colour and with the correct antigen distribution. One of the criticisms of IF is that it is labor intensive and requires highly skilled staff for the reading the specimen.
 |
 |
 |
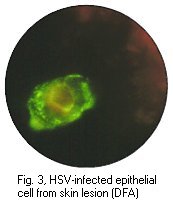
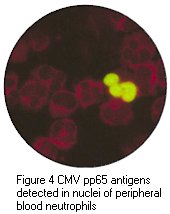
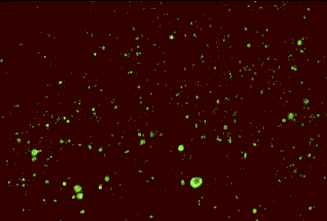
Positive immunofluorescense tests of HSV antigen from epithelial cell and CMV pp65 antigen from peripheral blood neutrophils. (Virology Laboratory, Yale-New Haven Hospital). Right: Positive immunofluorescense test of rabies virus antigen (CDC)
Detection of viral antibodies
IF is probably the simplest serological assay to set up. It simply requires virally infected cells that express viral antigens and a fluorescein-labelled antiserum against human immunoglobulin. IF can be used to detect IgG, IgM and IgA. Being very easy to set up, it is often the first and only serological assay available for newly discovered viruses, in particular arboviruses. IF is extensively used for the diagnosis of EBV infections and is also routinely used for other viruses such as VZV.