Togaviridae of Arthropod-Borne Viruses Infection
Alphaviruses
The Togaviridae comprises of 4 genera: Alphavirus, Rubivirus, Pestivirus, and Arterivirus. The only member of the rubivirus genus is rubella. The Pestivirus genus contains 3 viruses of veterinary importance; bovine diarrhoea virus, hog cholera virus, and border disease virus. The arterivirus genus contains a single member; equine arteritis virus. The alphavirus genus has 25 members of which 11 are recognized to be pathogenic for man. All alphaviruses are mosquitoe-borne and are geographically distributed mainly in the new world.
A. Properties
Belong to the family of Togaviridae
ssRNA enveloped viruses, icosahedral symmetry
virions 60-65nm in diameter
positive stranded RNA genome. A 26S subgenomic mRNA which represents the 3' one third of the genome codes for structural proteins. Translation of the whole genome from the 5' end produces non-structural proteins
alphaviruses are classified into 6 antigenic groups on the basis of neutralization tests and molecular biology
B. The American Equine Encephalitides
The alphavirus genus contains 4 viruses which produce encephalitis; EEE, WEE, VEE and Everglades. EEE, WEE and VEE were first isolated from the brains of dead horses in the 1930s. Focal epidemics of EEE have occurred from time to time in the Eastern USA, WEE is endemic in the Western USA, VEE is endemic in S.America and occasionally causes disease in N. America. The Everglades virus is related to EEE but is restricted to the state of Florida in the U.S.A and only 5 cases of encephalitis associated with it has been reported, despite a high seroprevalence rate in S. Florida (27%). EEE and WEE are maintained in nature between mosquitoes and birds, VEE between mosquitoes and rodents. The strain of VEE pathogenic for man are mainly amplified in horses, producing equine disease prior to the beginning of human disease. This is in contrast to the EEE and WEE viruses, where horses appear to be a dead end of infection.
The clinical manifestations may vary from an inapparent or a mild influenza like illness to the syndrome of encephalitis. The probability of developing encephalitis vary widely between the different viruses; it is highest for the EEE and lowest for the VEE. The mortality rate of encephalitis vary from 5% for WEE, 35% for VEE to 50% for EEE. Laboratory diagnosis may be made by the isolation of the virus from the brain, blood, CSF or throat washings. The viruses can be isolated using suckling mice or a variety of vertebrate cell lines such as Vero, where they produce a CPE, or using mosquito cell lines, where no CPE is seen. Alternatively, a serological diagnosis may be made for which a variety of tests are available.
No specific therapy is available against these viruses.
Although inactivated vaccines have been prepared by the U.S. army
against these viruses, they are not available for issue to the
public at large and their efficacy had yet to be proven.
Prevention depends on the surveillance of these viruses in
mosquitoes, birds, rodents and horses. Public warnings should be
issued when these viruses are prevalent. Vector control is
indicated in areas with a high mosquito population.
C. Alphaviruses Producing Fever, Polyarthritis, and Rash
Chikungunya Virus
The best example of a old world alphavirus is Chikungunya virus. It is found in areas of Africa and Asia, especially jungle areas. There are 3 possible modes of transmission, the first mode is sporadic cases arising when man come in contact with the jungle areas where the reservoir is in monkeys. Chikungunya is also prevalent in Thailand, where people to people spread occur by mosquitoes. The third type of Chikungunya exists in India, when during the rainy season, the population of mosquitoes increases greatly and epidemics can occur.
The clinical disease is a flu-like fever of acute onset, followed by a pharyngitis, maculopapular rash and arthritis. Minor haemorrhagic manifestations are occasionally seen. The diagnosis of infection is usually made by serology.
There are several other alphaviruses which produces a similar picture to that of Chikungunya virus;-
The Bunyaviridae family comprises more than 200 named viruses. Membership is usually based on antigenic interrelatedness or morphological similarity. The family is divided into 5 genera;
- Bunyavirus Bunyamwera, La Cross, Tahyna virus, transmitted mainly by mosquitoes
- Phlebovirus Sandfly fever, Rift valley fevers, transmitted by sandflies
- Uukuvirus transmitted by ticks, not associated with human disease
- Nairovirus Crimean-Congo Haemorrhagic Fever, transmitted by ticks
- Hantavirus Hantaan Virus, transmission does not require insects
Bunyaviruses, with the exception of Hantaviruses, are thought to be transmitted in nature by arthropods; most frequently mosquitoes but occasionally phlebotomine sandflies, midges and ticks. Vertebrate reservoirs have been demonstrated for some viruses. In others, tranovarial transmission is thought to play a dominant role in virus maintenance. Man is not known to be a natural or reservoir for any of these viruses, with the probable exception of sandfly fever.
A. Properties
Enveloped ssRNA viruses, virions 100 nm in diameter
5-10 nm projections visible on the surface
genome consists of 3 pieces of negative stranded RNA (the small RNA of phlebovirus is ambisense)
virion has 2 surface glycoproteins G1 and G2, with HA and virus neutralization epitopes
uncertain whether reassortment takes place is an uncommon event
B. Bunyavirus Genus
C. Phlebovirus Genus
There are at least 45 members which are mostly associated with sandflies. These viruses do not usually replicate in mosquitoes. There is evidence for transovarial transmission of many phleboviruses.
D. Uukuvirus_Genus
Members of this genus have been found in ticks but they have
yet to be associated with any human disease, even though
antibodies against these viruses have been demonstrated from
Czechoslovakia, Hungry and Norway.
E. Nairovirus Genus
All known members of the Nairovirus genus are thought to be transmitted by ticks and have often shown to be vertically transmitted. Their ecology is not completely understood.
Crimean-Congo Haemorrhagic Fever - During the Second
World War, 200 cases of severe haemorrhagic fever was described
from the Crimea. Similar diseases have been recognized from the
USSR and parts of Eastern Europe. A similar disease was
subsequently described in the Congo. It is now clear that a
single antigenic strain of the virus is distributed from the
sub-Saharan Africa, Eastern Europe, Middle East and possibly
India. The virus is carried by ticks where transovarian
transmission have been demonstrated. Certain vertebrate hosts are
also infected, such as hedgehogs, hares and domestic animals,
where amplification is known to occur. Medical personnel are at
some risk of secondary infection and accidental parenteral
inoculation is dangerous. Following an incubation period of 3 to
21 days, a non-specific febrile illness of abrupt onset develops.
The second phase of the illness sees the development of
haemorrhagic manifestations which lasts several days. Petechiae,
GI haemorrhage, haematuria may be seen as are marked neurological
manifestations. Hepatosplenomegaly may be present as well as
bradycardia, hypotension and in some sever cases, shock. A
recovery phase then follows. Mortality rates of 10-40% had been
reported. The virus may be readily isolated from the blood by the
intracranial inoculation of suckling mice, which rapidly die of
paralytic disease. A CPE is also seen in vero cells and other
cell cultures can be used. Treatment is mainly supportive. The
drug ribavirin has encouraging results in vitro and systemic
therapy with this agent should be considered in severe cases.
Hyperimmune convalescent serum may be useful and those with a
high titre of virus neutralizing antibodies should be selected.
An inactivated vaccine had been prepared. Although it has few
side effects, it does not appear to elicit a high titre of virus
neutralizing antibodies.
F. Laboratory Diagnosis Bunyavirus Infections
The viruses of the family Flaviviridae are important
arthropod-borne viruses in both human and veterinary medicine.
They are transmitted by mosquito and ticks and usually are
maintained in a transmission cycle in nature. They are widely
distributed throughout the world with the exception of the polar
region, although a specific flavivirus may be geographically
restricted to a continent or a particular part. They produce a
broad spectrum of clinical responses in humans ranging from
asymptomatic infection to fulminant encephalitis or haemorrhagic
fever. Nearly 60 flaviviruses are known to exist but many are yet
to be shown to cause disease in humans.
A. Properties
ssRNA enveloped viruses, 40-50 nm in diameter
positive RNA genome of around 10,000 nucleotides
3 structural proteins (an envelope glycoprotein, a nucleoprotein, and a small membrane protein), and a number of non-structural proteins.
one single reading frame, no subgenomic mRNA
classified in terms of cross-reactivity and the host.
The flavivirus family is divided into 7 subgroup of viruses. The antigenic determinants are carried by the envelope glycoprotein which is recognized by both HI and neutralization tests. 3 types of antigenic determinants are found for flaviviruses;
The envelope glycoprotein contain mainly type-specific
determinants and to a lesser extent complex-reactive and
group-reactive determinants. The nucleocapsid proteins had only
been shown to contain group-reactive determinants.
B. Flaviruses Producing Encephalitis
The flavivirus family contains many viral agents which produces encephalitis. Flavivirus encephalitides are either mosquito- borne, tick-borne, or have an unknown vector.
Geographical
Mosquito-borne
St Louis encephalitis birds Americas, Caribbean
Japanese encephalitis birds, pigs N and SE Asia, India
Murray
Valley
birds
Australia
encephalitis
West Nile birds Africa, Asia, Europe
Ilheus birds (humans?) Central and South America
Tick-borne
Russian
spring-rodents,
birds
Russia
summer encephalitis
Central
European
rodents,
birds,
Western and Central Europe
encephalitis
goats
Louping ill rodents, sheep Great Britain
Powassan Squirrels, chipmunks N. America, USSR
No known vector
Rio bravo bats Americas
Rocio birds (humans?) Brazil
Negishi
unknown
Japan
C. Mosquito-Borne Flaviviruses
St. Louis encephalitis occurs in endemic and epidemic form throughout the Americas and is the most important arboviral disease of North America. It is closely related to Japanese encephalitis and the Murray Valley encephalitis viruses. From 1955 to 1988, over 5000 cases of SLE have been reported to the Centers for Disease Control. The reported cases are only a fraction of those that actually occur. The largest epidemic occurred in 1975 when 1815 cases were reported. The virus is maintained in nature by a bird-mosquito-bird cycle.
The incubation period is 21 days. The ratio of inapparent to apparent infection ranges from 16:1 to 425:1. Children are much more likely to have inapparent infection than adults. The morbidity and mortality rate increases with age. Patients who are symptomatic will usually present with or progress to one of three syndromes (1) febrile headache (2) aseptic meningitis (3) encephalitis. The laboratory diagnosis is usually made by serology. Treatment is supportive and no vaccine is available.
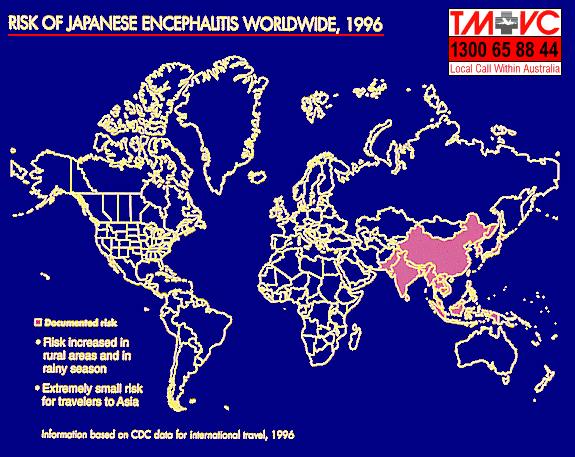
Japanese encephalitis is a major public health problem in Asia, SE Asia, and the Indian subcontinent. Prior to 1967, thousands of cases with several hundred deaths were reported each year. In endemic areas where vector control and vaccination had been undertaken, the incidence had dropped dramatically. Epidemics had been reported from Japan, China, Korea, Taiwan, USSR, Vietnam, Philippines, ASEAN countries, India and Bangladesh. The transmission cycle in nature involve the Culex and Aedes mosquitoes and domestic animals, birds, bats, and reptiles. Man is not a preferred host for Culex mosquitoes and transmission of JE virus does not usually occur until mosquito populations are large.
Japanese encephalitis produces a high inapparent to apparent infection ratio, ranging from 25:1 to 500:1 case of encephalitis. However, when encephalitis occur, the mortality rate is in the range of 20 to 50%. Some patients will only show an undifferentiated febrile illness or have mild respiratory tract complaints. The diagnosis is usually made serologically as virus isolation is not usually successful. No specific treatment is available. An inactivated suckling mouse brain vaccine had been available since the early 1960s which had been extensively used throughout Asia. The efficacy rate ranges from 60 to 90%. Despite the vaccine being a mouse brain preparation, no postvaccination demyelinating allergic encephalitis had been reported. Mild symptoms occur in 1% of vaccinees and thus the vaccine is generally to be considered as safe.
3. Murray Valley Encephalitis
This virus is closely related to Japanese encephalitis and resembles JE clinically. It is confined to the Australia and New Guinea, where it is an important cause of epidemic encephalitis periodically. In the 8 epidemics that took place between 1917 to 1988, 330 cases were reported in Australia. The diagnosis is made by serology and no specific treatment or vaccine is available.
4. West Nile Fever
West Nile fever is a dengue-like illness that occurs in both epidemic and endemic forms in Africa, Asia, and the Mediterranean countries. Areas of high endemicity include Egypt and Iran where most of the adult population will have antibodies. West Nile virus is a member of the St Louis encephalitis complex and is transmitted by Culex mosquitoes. The virus is maintained in nature through a transmission cycle involving mosquitoes and birds. Children will usually experience an inapparent or a mild febrile illness. Adults may experience a dengue-like illness whilst the elderly may develop an encephalitis which is sometimes fatal. The diagnosis is usually made by serology although the virus can be isolated from the blood in tissue culture. No vaccine fro the virus is available and there is no specific therapy. Among the arboviruses, it is difficult to distinguish clinically between West Nile, dengue and Chikungunya. In the absence of a rash, a number of toga and bunyaviruses should also be considered in the differential diagnosis.
5. Ilheus Virus
This virus is found in Latin America where it causes a febrile
illness with arthralgia. Occasionally a mild encephalitis is
seen. The virus can often be confused with dengue, St Louis
encephalitis, yellow fever and influenza viruses.
II. Tick-Borne Encephalitis Viruses
Tick-borne encephalitis viruses occur in temperate climates of Western and Eastern Europe and the USSR. These viruses are so closely related antigenically that it is uncertain whether to group them as separate viruses or as variants of the same virus. TBE viruses can be transmitted to a wide range of animals by ticks and is probably maintained in nature by small rodents. Humans can be infected via tick bites or by drinking milk of infected animals such as goat, cows and sheep. The clinical presentation vary from asymptomatic infection to fulminant encephalitis and death. The diagnosis is made serologically. By the time overt clinical manifestations are seen, the viraemia had already subsided so that the virus cannot be isolated from the blood or CSF. The treatment of TBE is supportive. A formalin-inactivated vaccine is available for use in the USSR which is recommended for persons living in endemic areas and for laboratory workers who may be exposed to the virus.
Louping-ill
Louping ill is primarily a disease of sheep in England,
Ireland and Scotland. Cattle, pigs, deer and some small mammals
and ground-dwelling birds are also infected. It is relatively
rare disease of humans caused by contact with infected tissue of
sheep (butchers and vet), laboratory accidents and through tick
bites. The disease caused resembles that of a mild form of
tick-borne encephalitis. The disease starts of with a mild
influenza-like illness which may proceed to a mild
meningoencephalitis. A vaccine is available for sheep which
should reduce human disease.
C. Flaviruses_Producing_Haemorrhagic_Fever
a. Epidemiology
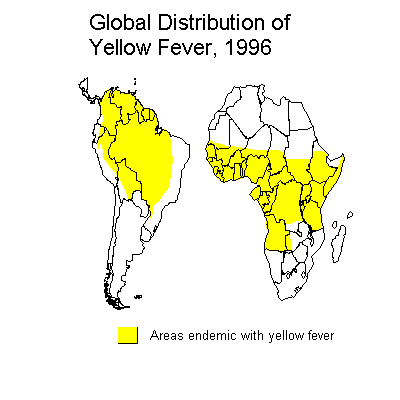
Yellow fever, once a scourge of the port cities of North America and Europe, remains an important endemic and epidemic disease of Africa and South America. Yellow fever occurs in 2 major forms: urban and jungle (sylvatic) yellow fever. Jungle YF is the natural reservoir of the disease in a cycle involving nonhuman primates and forest mosquitoes. Man may become incidentally infected on venturing into jungle areas. The S American monkeys are more prone to mortality once infected with YF than the old world monkeys, suggesting that American YF probably originated from the old world as a result of sailing ships.
The urban form is transmitted between humans by the Aedes aegypti mosquito and thus the potential distribution of urban YF is in any areas where infestation with Aedes aegypti occurs, including Africa, S and N America and Asia. Although the urban vector is present in Asia, yellow fever has never been established there. The majority of reported human YF cases come from Africa (Angola, Cameroon, Gambia, Ghana, Nigeria, Sudan, and Zaire) and S America (Brazil, Bolivia, Columbia, Peru, Ecuador and Venezuela). Both of these continents have jungle yellow fever transmitted in a monkey-mosquito-monkey cycle. In these areas, YF is reintroduced into urban populations from time to time as a result of contact with jungle areas. YF cases occur more frequently at times of the year when there are high temperatures ad high rainfall, conditions which are most conducive to mosquito reproduction.
Once infected, the mosquito vector remains infectious for life. transovarial transmission of Aedes aegypti had been demonstrated and may provide a mechanism for the continuation of the jungle or urban cycle. Once the virus is inoculated into human skin, local replication occurs with eventual spread to the local lymph nodes and viraemia occurs. The target organs are the lymph nodes, liver, spleen, heart, kidney and foregut.
b. Clinical Features
The incubation period varies from 3 to 6 days, following which
there is an abrupt onset of chills, fever, and headache.
Generalized myalgias and GI complaints (N+V) follows and signs
may include facial flushing, red tongue and conjunctival
injection. Some patients may experience an asymptomatic infection
or a mild undifferentiated febrile illness. After a period of 3
to 4 days, improvement should be seen in most patients. The
moderately ill should begin to recover, however, the more
severely ill patients with a classical YF course will see a
return of fever, bradycardia (Faget's sign), jaundice, and
haemorrhagic manifestations. The haemorrhagic manifestations may
vary from petechial lesions to epitaxis, bleeding gums, GI
haemorrhage (black vomit of YF). 50% of patients with frank YF
will develop fatal disease characterized by severe haemorrhagic
manifestations, oliguria and hypotension. Frank renal failure is
rare. Rarely, other clinical findings such as meningoencephalitis
in the absence of other findings have been described.
c. Laboratory Diagnosis
The differential diagnosis of YF include typhoid, leptospirosis, tick-borne relapsing fever, typhus, Q fever, malaria, severe viral hepatitis, Rift valley fever, Crimean-Congo haemorrhagic fever, Lassa, Marburg and Ebola fever. Yellow fever can be diagnosed serologically or by virus isolation. The serological diagnosis can be made by HI, CF and PRN tests. Virus isolation can be attempted from the blood which should be obtained within the first 4 days of illness. A variety of techniques are available for virus isolation, such as intracerebral inoculation of newborn Swiss mice or inoculation into Vero, LLC MK-2, BHK, or arthropod cell lines.
d. Treatment and Prevention
No specific antiviral therapy is available and treatment is
supportive. Intensive medical treatment may be required but this
is difficult to provide as many epidemics occur in remote areas.
Yellow fever is regarded as a quarantinable disease of
international public health significance and public health
officials should be notified as soon as possible so that vector
eradication and mass immunization can be carried out as soon as
possible to prevent an epidemic. A live attenuated vaccine known
as the 17-D had been available since 1937. The vaccine is
regarded as highly effective and generally safe, with mild
reactions such as headache, myalgia and low grade fever occurring
in 5 to 10% of vaccinees. Vaccination is recommended for
residents of endemic areas and should be included in routine
vaccination programs. Travelers to endemic areas should also be
vaccinated. It is officially recommended that a booster dose
should be given every 10 years although this may change in view
of recent data on the long persistence of YF antibodies. The
contraindications to the use of 17-D vaccine are pregnancy,
altered immune states, and hypersensitivity to eggs.
2. Kyasanur Forest Disease
This is a tick borne disease closely related to the tick-borne
encephalitis complex and is geographically restricted to
Karnataka State in India. Haemorrhagic fever and
meningoencephalitis may be seen. The case-fatality rate is 5%.
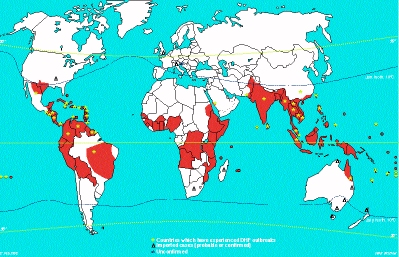
Hundreds of thousands of cases of dengue occur every year in
endemic and epidemic forms in tropical and subtropical areas of
the world. The attack rates during epidemics can reach as high as
50%. Dengue is a prevalent public health problem in SE Asia, the
Caribbean, Central America, Northern South America and Africa.
(See red coloured areas in map below). In hyperendemic areas,
most cases occur in young children as the majority of the
population had already been infected with multiple serotypes. In
other areas, older children and adults are more likely to be
affected. Maximum number of cases occur during the months of the
year with the highest rainfall and temperatures, when Aedes
aegypti populations are at their highest. A. aegypti mosquitoes
deposit their eggs in waterfilled containers and thus
reproduction is highest during periods of high rainfall.
4 serologically distinguishable types of dengue are recognized (DEN 1-4). The vector mosquito becomes infected by feeding on a viraemic host. The virus becomes established in the salivary glands of the mosquito from where it can be transmitted to susceptible individuals. Following an incubation period of 2 to 7 days, the virus is disseminated (route unknown), to the organs of the RE system (liver, spleen. bone marrow and lymph nodes). other organs may be involved such as the heart, lungs and GI tract.
a. Clinical Manifestations
The clinical presentation of dengue in children is varied. The disease may be manifested as an undifferentiated febrile illness, an acute respiratory illness, or as a GI illness: atypical presentations which may not be recognized by clinicians as dengue. Older children and adults infected with dengue the first time will display more classical symptoms: sudden onset of fever, severe muscle aches, bone and joint pains, chills, frontal headache and retroorbital pain, altered taste sensation, lymphadenopathy, and a skin rash which appears 3 days after the onset of fever. The rash may be maculopapular, petechial or purpuric and is often preceded by flushing of the skin. Other haemorrhagic manifestations may be seen such as epitaxis, gingival bleeding, ecchymoses, GI bleeding, vaginal bleeding and haematuria. Severe cases of bleeding should not be diagnosed as Dengue haemorrhagic fever (DHF) or Dengue shock syndrome (DSS) unless they meet the criteria below.
DHF or DSS are usually seen in children and usually occurs in 2 stages. The first milder stage resembles that of classical dengue and consists of a fever of acute onset, general malaise, headache, anorexia and vomiting. A cough is frequently present. After 2 to 5 days, the patient's condition rapidly worsens as shock begins to appear. Haemorrhagic manifestations ranging from petechie and bleeding form the gums to GI bleeding may be seen. An enlarged nontender liver is seen in 90% of cases. The WHO recommended the following criteria for the diagnosis of DHF and DSS:
1. Fever
2. Haemorrhagic manifestations including at least a positive
tourniquet test.
3. Enlarged liver
4. Shock
5. Thrombocytopenia (<=100,000 ul)
6. Haemoconcentration (HcT increased by =20%)
A diagnosis of DSS is made when frank circulatory failure is
seen, and occurs in one third of cases of DHF. DHF has been
graded by the WHO on the basis of its severity.
| Grade I Grade II Grade III Grade IV |
Fever accompanied by
non-specific constitutional symptoms, the only
haemorrhagic manifestation is a positive tourniquet
test Spontaneous bleeding in addition to the manifestations of Grade I patients, usually in the form of skin and/or other haemorrhages Circulatory failure manifested by rapid and weak pulse, narrowing of pulse pressure (20 mmHg or less) or hypotension, with the presence of cold clammy skin and restlessness Profound shock with undetectable blood pressure and pulse |
| Grade III and Grade IV DHF are also considered as Dengue Shock Syndrome. |
b. Immunology
The immunological response to dengue infection depends on the individual's past exposure to flavivirus. The flavivirus group shares cross-reacting antigen(s). Primary infection results in the production of antibodies against the infecting serotype predominantly. Reinfection with another dengue serotype (or other flaviviruses) usually produces a secondary (heterotypic) response characterized by very high titres to all 4 dengue virus serotypes and other flaviviruses, so that serological identification of the infecting agent is quite difficult if not impossible. After a first infection with one dengue serotype, cross-immunity to other serotypes may persists for a few months, but after 6 months, reinfection with another serotype may occur.
c. Pathology
There are 2 theories proposed for the pathogenesis of DHF and DSS: virus virulence and immunopathological mechanisms. The weight of the available evidence supports the immunopathological theory. DHF and DSS occurred most often in patients with a secondary (reinfection) serological response. However, the observation that DHF and DSS occurred in infants with a primary response cast some doubt on this theory until it was demonstrated that preexisting maternal antibody had a similar effect to acquired antibody. The antibody-dependent theory proposes that in the presence of non-neutralizing heterotypic antibody (whether maternally derived or not) to dengue, virus-antibody complexes are formed which are more capable of infecting permissive mononuclear phagocytes than uncomplexed dengue virus.
d. Laboratory Diagnosis
1. Serology - HI, CF and PRN tests are commonly used. The high degree of cross-reactivity between flaviviruses can make the interpretation of serological results very difficult.
2. Virus isolation - this can be accomplished by the intracerebral inoculation of sera from patients into suckling mice. Sera can also be inoculated intrathoracically into Aedes mosquitoes. Head squash preparations are examined for the presence of antigen by the FA technique. Cell cultures such as LLC MK-2 and several mosquito-derived cell lines can be used.
e. Treatment and Prevention
There is no specific antiviral treatment available. Management
is supportive and intensive medical management is required for
cases of severe DHF and DSS. No vaccine for dengue is available
but a tetravalent live-attenuated vaccine has been evaluated in
Thailand with favourable results. Scale-up preparation for
commercial production of the vaccine is underway and it is
anticipated that the vaccine will become available soon and
evaluated in large scale clinical trials. To avoid dengue,
travelers to endemic areas should avoid mosquito bites.
Prevention of dengue in endemic areas depends on mosquito
eradication. The population should remove all containers from
their premises that may serve as vessels for egg deposition.
Vector surveillance is an integral part in control measures to
prevent the spread of dengue outbreaks. Regular inspections as
part of law enforcement may be used in the control of mosquito
vectors. The object of source reduction is to eliminate the
breeding grounds in and around the home environment, construction
sites, public parks, schools and cemeteries. Illegal dumping of
household refuse provide favorable breeding sites for mosquitoes.
Long-term control should be based on health education and
community participation, supported by legislation and law
enforcement. Domestic water supplies should be improved in order
to reduce the use of containers for the storage of water.
ORBIVIRUSES
Orbiviruses are insect-borne viruses primarily of veterinary importance. Orbiviruses contain dsRNA arranged in 10 segments except for the members of the Colorado tick fever serogroup which have 12 segments of dsRNA. They vary in size from 50 to 90nm with 92 capsomers. The capsid is a double-layered protein. The outer coat is diffuse and unstructured while the inner layer is organized in pentameric-hexameric units. Colorado tick fever is caused by a virus belonging to the family of Reoviridae. It is a zoonotic disease of rodents and is transmitted to man via tick bites. It is prevalent in the Rocky Mountains and more western regions of the USA. It is a dengue-like illness albeit with a relatively low incidence. Colorado annually reports 100 to 300 cases but the disease is underreported there and from other western states as well. There is a strong seasonal trend with the majority of cases occurring between February and July. Chipmunks and squirrels serve as amplifying hosts.
Following an incubation period of 3 to 6 days, a high fever of acute onset is seen, along with chills, joint and muscle pains, headache, N+V. A maculopapular rash may be seen in a minority of patients. A more severe clinical picture may be seen in children, who may develop haemorrhagic manifestations including severe GI bleeding and DIC. Aseptic meningitis or encephalitis may be seen. CTF may be diagnosed by virus isolation whereby the patient's blood is inoculated into suckling mice or cell culture lines such as Vero, followed by identification by IF, N or CF tests. More rapid diagnosis can be made by performing IF directly on the blood clots. A IgM tests as well as other serological techniques are available for serological diagnosis. No licensed vaccine for CTF is available nor is it practicable because of the rarity and the benign nature of the disease. Public health education remains the most preventative measure.