CYTOMEGALOVIRUS
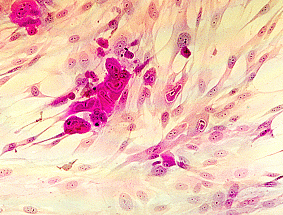
Cytomegalovirus is named after the appearance of its cytopathic effect in cell culture. In addition, CMV produces typical owl's eye intranuclear inclusion bodies in infected cells.
Properties
A member of the herpesvirus family
ds DNA enveloped virus
Nucleocapsid 105nm in diameter, 162 capsomers
2 additional forms are seen in cell culture ;
(i) "dense body", which does not contain DNA or nucleocapsid
(ii) "non-infectious enveloped particle" (NIEP), consists of an empty capsid surrounded by a lipid envelope.
The structure of the genome of CMV is similar to other herpesviruses, consisting of long and short segments which may be orientated in either direction, giving a total of 4 isomers.
A large no. of proteins are encoded for, the precise number is unknown.
Some areas of the CMV genome are homologous with regions of human chromosomal DNA. This means that probes for CMV DNA must be carefully evaluated before being to used to diagnose CMV infection in human cells. Also, this has fueled speculation that CMV may be oncogenic although there is no concrete evidence to date. The genome also contains a gene with striking homology to class I HLA molecules, although its function remains to be defined. The CMV DNA can also be digested with restriction endonucleases so that, following electrophoresis, oligonucleotide patterns characteristic of different strains can be produced. Although this technique cannot totally prove that 2 strains of CMV are identical, it does, however produce useful epidemiological information. There is no evidence to suggest that any one strain is associated with any particular type of clinical disease.
In the urine and probably other bodily fluids, CMV is
physically associated with host ß2-microglobulin, which does
appear to have a protective effect on the virus against host
immunoglobulins. Since ß2m normally binds to HLA-I molecules,
this suggests that CMV may use such cell surface molecules as
receptors. The only cells fully permissive for CMV infection in
vitro are human fibroblasts, whereas in vivo, cells of all types
can be infected. Later in the infection, CMV induces the
appearance in infected fibroblasts of a Fc receptor which has a
high affinity for human IgG. This has produced many problems in
the past for serological methods using immunofluorescence as all
human sera bind avidly to CMV infected cells. However the problem
is overcome by the use of mouse monoclonal antibodies as the
latter is not bound by the Fc receptor. The advantage of inducing
the production of a Fc receptor is unknown, the production of Fc
receptors in vivo may allow opsonized bacteria to gain access to
cells which they do not normally infect and may aid the entry of
opsonized HIV. Unlike HSV, CMV does not switch off host
macromolecular synthesis but actually stimulates DNA, RNA and
protein synthesis.
EPIDEMIOLOGY
CMV is one of the most successful human pathogens, it can be
transmitted vertically or horizontally usually with little effect
on the host. Transmission may occur in utero, perinatally or
postnatally. Once infected, the person carries the virus for life
which may be activated from time to time, during which infectious
virions appear in the urine and the saliva. Reactivation can also
lead to vertical transmission. It is also possible for people who
have experienced primary infection to be reinfected with another
or the same strain of CMV, this reinfection does not differ
clinically from reactivation although it may be important
epidemiologically to distinguish between reactivation and
reinfection. The term recurrent infection can be used to cover
both reinfection and reactivation. In developed countries with a
high standard of hygiene, 40% of adolescents are infected and
ultimately 70% of the population is infected. In developing
countries, over 90% of people are ultimately infected.
Routes_of_transmission
1. Intrauterine ;- Intrauterine infection is thought to follow maternal viraemia and placental infection. Research into intrauterine infection has been hampered by the lack of maternal symptoms. Intrauterine transmission only occurs in 1/3 rd of women who experience primary infection during pregnancy. Congenital infection can also result from recurrent maternal infection. Intrauterine transmission can occur at any time during pregnancy. (reinfection or reactivation). This may be surprising in view of the fact that large amounts of anti-CMV antibodies may be in circulation. However there may be other routes available for transmission such as the introduction of infected spermatozoa into the uterus or the reactivation of CMV that is already present in the endometrium of the uterus. However, such routes are purely speculative at the present.
2. Perinatal ;- Perinatal infection is acquired mainly through 2 sites - (i) Infected genital secretions, or (ii) Breast milk. Once ingested, CMV usually gains access to the neonate by infecting the salivary glands. Overall, 2 - 10% of infants are infected by the age of 6 months worldwide. Perinatal infection is thought to be 10 times more common than congenital infection.
3. Postnatal ;- The usual absence of symptoms associated with CMV infection made it difficult to ascertain routes of transmission. The following are the probable routes of transmission ;-
(1) Saliva - saliva is probably the main route through which the virus is transmitted postnatally. This is likely to be the route through which the virus is transmitted amongst young children.
(2) Sexual transmission - sexual transmission is possible but not proven beyond doubt. CMV is found in semen and in the cervix. However oral-oral contact frequently occurs before intercourse which may well be the route through which the virus has been transmitted after intimate contact. However, susceptible homosexuals run a particularly high risk of being infected. Rectal intercourse seems to be a risk factor.
(3) Blood transfusion - In the early 1960s, a postperfusion syndrome was recognized in open heart surgery patients who received large quantities of blood which were contaminated with CMV. However transmission of CMV by blood is clearly a rare event as only 3 - 5% of blood taken from seropositive donors leads to infection of seronegative recipients. It has not been possible to determine which donors have a high risk of transmitting the virus. It is possible that CMV persists in a latent state in blood leucocytes, and CMV is occasionally reactivated on transfusion.
(4) Organ transplantation - Patients undergoing renal transplantation are particularly at risk. Seronegative recipients have a 5% chance of acquiring primary infection from seronegative donors compared to a chance of 70 - 80% of acquiring primary infection from seropositive donors. It seems that CMV is transmitted via infected cells in the donor kidney.
It is also possible to transmit CMV in other types of organ transplantation such as the bone marrow, heart, heart and lung, and the liver. Although in the case of the liver, 50 - 100 units of blood is commonly given which carries a far greater risk of transmission. Often, particularly in the case of bone marrow transplantation, the virus causing disease after the transplantation came from the recipient rather than the donor. Also the serological evidence for the transmission of CMV in the case for bone marrow transplantation is not as strong as that for renal transplantation.
Mechanism_of_Virus_Recurrence
In the general adult population, recurrent CMV infection is uncommon since very few seropositive individuals seem to be excreting virus from the saliva or urine at any time. Where multiple isolates have been obtained and typed by restriction endonuclease analysis, they usually appear to be the same strain which suggests reactivation of the latent virus, though reinfection by the same strain cannot be ruled out. Where the strains are different, reinfection may have occurred or although 2 latent strains of CMV may be reactivated at the time. Immunosuppression produces as increased state of CMV isolation which is usually due to reactivation. Recurrent CMV infection in adults seem to be age dependent. Studies of pregnant and non-pregnant women as well as male homosexuals have shown that up to 10% of seropositive individuals may be excreting CMV form the saliva, urine or the cervix in the case of women. Excretion rates are however, very low after the age of 30 years, suggesting that a more mature host response for suppression may have developed at this age.
Immune_Responses
1. Humoral Response ;- CMV specific IgM antibodies are produced during the primary infection and persists for 3 or 4 months, but are not produced in recurrent infections in immunocompetent individuals. However, immunocompromised individuals may fail to produce IgM with primary infection and 1/3rd of them have IgM detectable with recurrent infection. CMV IgG antibodies are produced at the time of primary infection and persists lifelong. With intrauterine infections, both IgM and IgG are produced by the fetus but the fetal IgG response can only be detected as the passively acquired IgG from the mother is catabolized. Although there is evidence to suggest that the humoral response may be beneficial, eg. immunocompromised patients who fail to develop IgM run a high risk of developing disseminated infection, the exact role of the humoral response remain uncertain.
2. CMI ;- CMI is thought to play a key role in the suppression of CMI infection. The test most widely used to measure CMI is the lymphocyte transformation response (LTR) which measures the recognition, not the effector function of T lymphocytes. Most seropositive adults have a positive LTR, whereas few congenitally or perinatally infected infants can respond. This failure of LTR recovers with time and there is a direct correlation between cessation of viruria and acquisition of LTR responsiveness at 3 -5 years of age.
A._Congenital_Infection
The definition of congenital CMV infection is defined as ;- The isolation of CMV from the saliva or urine within 3 weeks of birth. After that, the virus may be transferred from the mother to the infant via breast milk. In the neonate CMV is now the commonest cause of congenital infection and affects around 0.3% - 1% of all live births. 5 - 10% of congenitally infected infants have symptoms at birth. Fatal disease occurs in 20% of these infants. 90% of the symptomatic survivors have sequelae and up to 15% of the asymptomatic survivors have sequelae. CMV is now the second commonest cause of mental retardation after Down's syndrome and causes more cases of congenital damage than rubella.
U.S.A. U.K.
No. of live births
p.a.
3,000,000
700,000
Rate of congenital
CMV
1%
0.3%
No. of infected
infants
30,000
2100
Symptomatic at birth (5 -
10%)
1,500 -
3,000
105
Fatal disease
(~20%)
300 -
600
22
No. with sequelae (90% of survivors) 1080 -
2160
83
Asymptomatic (90 - 95%
)
27000
1995
No. with late
sequelae
1350 -
4550
315
In the UK, 5% of congenitally infected babies are born with symptoms of "cytomegalic inclusion disease" and their prognosis is poor. The remaining 95% appear to be normal at birth but a proportion develop sequelae later on in life.
The classical presentation of cytomegalic inclusion disease is IUGR, jaundice, hepatosplenomegaly, thrombocytopenia and encephalitis with or without microcephaly. It is often difficult to differentiate on clinical grounds between several agents that cause intrauterine infection. The severe thrombocytopenia, hepatitis, pneumonitis and myocarditis may be life threatening. CNS involvement may lead to seizures, focal neurological signs and mental retardation. Unlike rubella, there is no evidence that CMV is teratogenic. Most of the damage is caused by the destruction of target cells once they have been formed and unlike rubella, the fetus can be damaged by infection during any stage of pregnancy. In 20% of cases ( 1% of those who are congenitally infected ), the infection is so severe that they die during infancy. The rest are likely to sustain serious abnormalities for the rest of their lives. Brain damage is by far the most common abnormality on follow-up and this may manifest as microcephaly, mental retardation, seizures, cerebral palsy. The spectrum of brain damage may vary from mild to profound. Optic atrophy, deafness and blindness may also be present. The following is a list of the effects of congenital CMV infection ;-
1. CNS abnormalities - microcephaly, mental retardation, spasticity, epilepsy, periventricular calcification.
2. Eye - choroidoretinitis and optic atrophy
3. Ear - sensorineural deafness
4. Liver - hepatosplenomegaly and jaundice which is due to hepatitis.
5. Lung - pneumonitis
6. Heart - myocarditis
7. Thrombocytopenic purpura
8. Haemolytic anaemia
9. Late sequelae - damage to the enamel forming organ of the teeth resulting in yellow discoloration of the teeth and brittleness. This occurs in 40% of such infants.
It has become apparent that late sequelae are seen in congenitally infected individuals who are asymptomatic at birth. 15% of such individuals are likely to have hearing defects and reduced intelligence compared to normal people. Like congenital rubella, progressive sensorineural deafness is seen.
B._Perinatal_Infection
Despite the continued excretion of high titres of virus for many months, the vast majority of perinatally infected infants do not develop acute symptoms although cases of infantile pneumonitis have been reported. This appears to be an exceedingly rare event.
C._Postnatal_Infection
The incubation period for CMV is thought to be 4 - 8 weeks. Primary CMV infection in the postnatal period is usually mild or asymptomatic. Occasionally, primary infection may be accompanied by the syndrome of infectious mononucleosis with atypical lymphocytosis. This is similar to the syndrome produced by EBV except that lymphadenopathy is uncommon and the Paul-Bunnel test is negative. The postperfusion syndrome is essentially CMV mononucleosis acquired by blood transfusion. Sometimes, the hepatitis picture predominates so that a diagnosis of non-A, non-B hepatitis is made.
Immunocompromised patients ;- Primary infection in immunocompromised individuals are far less likely to be asymptomatic. The following may be seen ;-
1. These patients develop a spiking pyrexia which resolves
within a few days. Some may develop a viraemia with a septicaemia
like syndrome in the presence or absence of hepatitis.
2. Pneumonitis may develop which carries a grave prognosis, with
80 - 90% mortality.
3. The virus may disseminate to involve the retina, causing a CMV
retinitis.
4. CMV may disseminate to the gut, where it may cause an
asymptomatic infection or ulceration or haemorrhage by the
erosion of nearby blood vessels.
5. CMV induced immunosuppressive syndrome - The patient becomes
unable to deal with opportunistic infections such as pseudomonas.
In this instance, the underlying nature of CMV infection is not
recognized by the clinical staff, who are naturally more concern
with the opportunistic infection.
In addition to these general clinical features other features may be found in patients with AIDS or renal transplant recipients ;-
Renal transplant patients may develop an interstitial glomerulonephritis following CMV infection. It has been described following primary and recurrent infection, is associated with viraemia and poor graft function and not to immune complexes. Allograft recipients typically have recurrent infection 2 - 3 months after the transplantation. On average, their viruria occurs one or two weeks later than that resulting from primary infection.
AIDS patients may develop a low grade encephalopathy
and CMV adrenitis. In addition, Kaposi's sarcoma is associated
with past CMV infection. Patients with KS are more likely to have
CMV IgG antibodies and at higher titres than the controls. Also
CMV DNA sequences have been found in KS tumours. However, a
recently discovered agent, HHV-8 is reported to have a much
firmer association with Kaposi’s sarcoma.
1. Virus Isolation ;- Urine, saliva, blood and biopsy samples can be used for virus isolation. Urine should be collected a sterile container without additives. Saliva samples should first be soaked on to a swab which is then broken off into transport medium. Blood should be collected into a heparinized bottle ( some phenolic preservatives found in proprietary pathology blood bottles may be toxic to blood cultures) containing 500 units of heparin. Tissue biopsies should be placed in sterile plastic containers. The specimens can be treated in the following ways ;-
(a) Cell culture - Human embryo lung fibroblasts are most commonly used. The specimen is inoculated into HEL cells and kept for 28 days with a blind passage at 14 days. CMV produces a typical focal cytopathic effect.
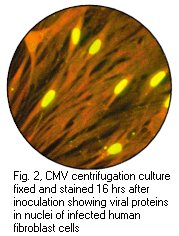
(b) DEAFF ( Detection of early antigen fluorescent foci ) ;-
This is a method used for the early diagnosis of CMV infection.
In immunocompromised patients, a sensitivity of 78% and a
specificity of 100% has been claimed. The specimen is inoculated
into cell culture which is examined 24 hours later by
immunofluorescence for expressed CMV encoded early proteins. The
monoclonal antibodies must be able to cover most, if not all
strains of CMV. Rapid culture methods other then the DEAFF tests
are also available.
(c) Histopathology - Cytomegalic inclusions can be recognized
from biopsy material by the typical "owl 's eyes appearance
"
(d) Tissue immunofluorescence - Infected lung and liver cells may be stained by specific anti-CMV antibodies. Broncheolavage specimens can also be examined in this manner. Results of high sensitivity and specificity are possible.
(e) Electron microscopy - Virions in the urine of congenitally infected infants may be visualized by EM in up to 80% of cases. However this is of no real value as rapid diagnosis is not required. In immunocompromised individuals though, the viral titres are generally lower than neonates and other herpesviruses are often present in the urine.
(f) ELISAs for CMV antigen in the urine - these tests carry low sensitivity as CMV is complexed to ß2-microglobulin in the urine.
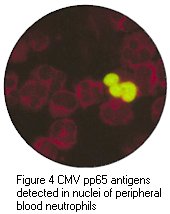
(g) CMV antigenaemia test - this test is based upon the detection of pp65, a structural protein expressed on the surface of infected polymorphonyclear leucocytes. The number of infected leucocytes present had been reported to correlate with the severity of infection. The main advantage of this test is that it is very rapid so that a result can be available within the same day. As a result, this test is now widely used especially in the monitoring of transplant recipients.
(h) Detection of CMV DNA by PCR - the use of PCR in the diagnosis of CMV infection had been widely studied. PCR offers the advantages of being rapid and sensitive. However, its inherent sensitivity poses a problem since latent CMV genomes, which are present in practically all seropositive individuals, may be detected. Therefore, it is critical to adjust the sensitivity of the PCR so that latent genomes are not detected. Real-time quantitative PCR is being increasingly used for the management of CMV infection in immunocompromised individuals. However the lack of standardized real-time PCR protocols hinders the comparison of the data across different centers and the development of uniform guidelines for the management of invasive CMV infections in immunocompromised individuals
 |
 |
 |
Cytopathic effect of CMV in cell culture (courtesy of Linda Stannard, University of Cape Town, S.A), Positive CMV DEAFF and pp65 antigenaemia tests (Virology Laboratory at Yale- New Haven Hospital)
2. Serology ;- CMV IgM antibodies are detected in primary
infection and lasts 3 - 4 months. It is not detectable in
recurrent infection except in immunocompromised patients where it
is detectable in about a third of the cases. CMV IgM may be
undetectable in primary infection in immunocompromised
individuals. Solid phase sandwich or antibody capture ELISAs or
RIAs are now in routine use. Interference by rheumatoid factor
should be excluded. CMV IgM can be sought for in the cord blood
samples from infants who are suspected of being congenitally
infected and the titre present is generally related to the outcome. However the
best method for diagnosing congenital CMV infection remains virus isolation.
CMV IgG is produced early in primary infection and persists lifelong. The detection of CMV IgG is useful as an "immune status screen" (Seropositive individuals are not protected from reactivation of reinfection). Rising titres of IgG can be used as markers of acute infection. This is particularly useful in diagnosing recurrent infections in normal individuals, and in immunocompromised patients who may not develop a IgM response to primary infection. Various methods are used for detecting CMV IgG including CFT, IFT, latex agglutination, ELISAs and RIAs. The test used at the RVL is a LA.
Where possible, serological investigation should be backed by virus culture, especially in the case of immunocompromised patients who may fail to mount an immune response. CMV IgG may also be transferred by blood products which may produce false positive results. The following are recommended methods for use in the diagnosis of CMV infection.
Site for virus
culture
Serology
Urine Saliva
Blood Tissue
affected
IgG IgM
Neonates + + - - - +
Adults + - + - + +
Pregnant women - - - - + +
Immunocompromised
+
+
+
+
+ -
A._Congenital_Infection
It is of utmost importance to make an unequivocal diagnosis. Babies with symptoms should be investigated with viral cultures of the urine, saliva and blood. IgG in cord blood is unhelpful as the IgG may be of maternal origin. CMV IgM can be detected in 90% of the cases. After the age of 3 weeks, a virological diagnosis of congenital infection cannot be safely made as perinatal infection may lead to the isolation of CMV and the development of IgM. Perinatal infection is 10 times as common as congenital infection. Clinical findings are of vital importance for such a diagnosis to be made.
Once a diagnosis is established, an estimate of the child's prognosis can be given from (1) the level of viruria, and (2) the level of antibodies in the sera. As for future pregnancies, the prognosis is extremely good as no cases of cytomegalic inclusion disease has ever been reported in consecutive siblings, therfore the risk of recurrence must be very low indeed.
B._Perinatal_Infection
Most cases are asymptomatic and undiagnosed. Cases of infantile pneumonitis should have urine, saliva and NPA cultured for CMV. The main importance of neonatal infection is the considerable difficulties it present for the diagnosis of congenital infection. Children with perinatal infection can infect adults. 10 - 20% of children in the classroom are asymtomatically excreting identical titres of CMV at any one time and it would be inappropriate to isolate such children.
C._Postnatal_Infection
Most cases of postnatal infection are asymptomatic and do not require management, except in cases of CMV mononucleosis or hepatitis, where appropriate supportive management may be indicated. Where primary infection occurred during pregnancy, due consideration should be given to termination of pregnancy.
Recurrent infections are invariably asymptomatic and no laboratory test is currently available which can detect which immune women are transmitting the virus in utero. Therefore it is not possible to contemplate STOP in these cases. Although congenital infection and cytomegalic inclusion disease (CID) can occur at any stage during pregnancy, CMV infection is most severe during the first trimester of pregnancy and so STOP may be justified if the diagnosis is made early enough. Natural termination of pregnancy is a common at this stage. The actual risks involved are still uncertain, the risk is thought to be in the region of 5% for the fetus developing cytomegalic inclusion disease after maternal infection in the first trimester. These results indicate that screening during early pregnancy would have little effect on the incidence of CID. There is also no evidence that symptomatic primary infection during pregnancy carries a significantly increased risk for CID than asymptomatic cases. There is little evidence that staff exposed professionally to infectious cases have an increased risk of contracting CMV infection but it seems prudent to advise pregnant staff to avoid such contacts if possible.
4. Immunocompromised Patients ;- It is essential to make the diagnosis of CMV infection early. Once extensive damage to target tissues has occurred, no antiviral therapy will be successful. Infections form the single commonest cause of death in allograft patients. CMV is the single most important infection in bone marrow recipients, responsible for 15% of deaths. The position with renal transplant patients is not as clear but CMV is clearly a major cause of mortality and morbidity. Surveillance cultures are routinely used in susceptible patients. Some centers collect weekly samples of urine, saliva, and heparinized from all allograft recipients and more frequently if the patient is unwell. If CMV is isolated from any site, the clinical state of the patient is reviewed with the view of decreasing or stopping immunosuppressive therapy. If the patient is asymptomatic or have a mild pyrexia, then no action is needed. If the patient is unwell, then immunosuppressive therapy should be decreased or stopped altogether. It is not clear whether drug therapy (many of the drugs are experimental) should be administered to all patients, CMV pneumonitis should certainly be treated but milder presentations need not be treated with drugs.
Ganciclovir remains the mainstay of treatment in severe CMV disease other than retinitis. Otherwise, valganciclovir is now the drug of choice for prophylaxis against CMV in solid organ transplant recipients. Forscarnet and cidofovir are used as second line drugs. There are also a number of drugs under investigation: Maribavir (UL97 kinase inhibitor), brincidofovir (oral bioavailable form of cidofovir), and letermovir (viral terminase complex inhibitor).
Prevention from CMV infection is desirable in 2 situations ;- (1) in preventing CID of the newborn and (2) infection in susceptible immunocompromised patients.
1. Prevention of Transmission ;- Seronegative renal allograft recipients should be matched to kidneys from seronegative donors. CMV matching has been shown to have a greater effect on the survival of the recipient than HLA class I Ag matching. However, reinfection from seropositive donor kidneys has been shown to cause disease in seropositive recipients. Therefore if seronegative kidneys are given to seronegative patients, then more infected kidneys would be given to seropositive recipients. Seronegative recipients should also receive blood from seronegative donors only.
2. Immunization ;- A live vaccine known as the Towne strain has been reported to be effective in conferring protection or in reducing the severity of disease in seronegative recipients given seropositive kidneys. It was also well tolerated and immunogenic but could not prevent reinfection of the recipients with a different strain of CMV. There were concerns about latency and reactivation of a live vaccine as well as potential oncogenicity. However, there was no evidence of reactivation in immunosuppressed patients or an excess of malignancies. Because of the possibility of reactivation, a live vaccine would be unacceptable for use in seronegative women. Therefore, subunit vaccines are currently being developed based on the membrane proteins SSK and GC1. One encouraging candidate is live vaccine using adenovirus as the vector. This vaccine can be given orally as in the case of adenovirus vaccines used for the protection against adult respiratory distress syndrome. This vaccine is currently being readied for clinical trials.
3. Periexposure Therapy ;- Valganciclovir or ganciclovir be given as prophylaxis against reactivation in susceptible seropositive transplant patients, in as much the same way as acylovir is given to seropositive patients at risk of reactivation of HSV ( when immunosuppressive therapy is given ). It has been reported that acyclovir itself may act prophylactically to reduce CMV infection and disease eventhough it has no role in treatment. Hyperimmune human immunoglobulin has been given in a few trials with encouraging results and also a-interferon has been reported to have some success in renal transplant patients. CMV-hyperimmune globulin and IVIG had been reported to be effective when given to seronegative recipients of solid organ transplants from seropositive patients.
4. Treatment ;- The treatment for an established infection is controversial at present. The drugs ganciclovir and foscarnet have been licensed for use in life threatening CMV infection in immunocompromised patients but have not been subjected to clinical trials. CMV lung infection in AIDS patients is not normally treated because they do not mount the CMI necessary for immunopathology. Immunoglobulin may be given allograft patients with CMV pneumonitis because the antibodies in the preparation may block the immunopathological response to antigens in the lung. The drugs used at present have serious interaction with other drugs eg. ganciclovir and AZT, foscarnet and pentamidine. Moreover, CMV strains resistant to pentamidine have been described.
Site of CMV
Type of patient Clinical condition detection Suggested treatment
Allograft None Blood Ganciclovir or foscarnet or cidofovir
Allograft or AIDS
G.I.
ulcer Biopsy
Ganciclovir or foscarnet or cidofovir
Retinitis
Not possible
Allograft Pneumonitis Lung/blood Ganciclovir and I.V.immunoglobulin
AIDS
Pneumonitis
Lung
None
CMV_infection_in_bone_marrow_transplant_recipients
Bone marrow transplantation is increasingly used as therapy for aplastic anaemia, various haematological malignancies, immunodeficiency syndromes and thalessaemia. The process involves the ablation of the host's haematopoietic and immune systems and replacement with those of the marrow donor. In BMT patients, every aspect of the immunological function is depleted. The most commonly used donor is a HLA matched sibling. However, related donors who are not fully matched and fully matched but unrelated donors have also been used. Major complications after transplantation include graft versus host disease (GvHD), toxicity from conditioning and infection. Immunosuppression is given for 100 days or more after allogeneic transplant to prevent GvHD, and patients with GvHD are treated with further immunosuppression. Both GvHD and its treatment increases the risk of infection.
BMT recipients are at increased risk of developing severe viral infections, in particular from viruses belonging to the herpesvirus family. CMV is the single most important infection in BMT recipients. CMV infection is the single greatest cause of failure of the transplant and occurs 1 to 3 months following transplantation. It occurs in 30 to 40% of seronegative patients given unscreened blood products and up to 80% of seropositive patients who reactivate latent virus (seropositive recipients of graft taken from seronegative donors are at higher risk than those receiving graft from seropositive donors) 15% of infected patients develop CMV pneumonitis which carries a mortality rate of almost 100% in the past. Once pneumonitis ensues, the clinical course is very rapid. Other manifestations of CMV infection include fever, oesophagitis, colitis, hepatitis, encephalitis retinitis, vasculitis, viraemia and bone marrow failure. The highest mortality occurs 2 to 3 months after the transplant when the patients is out of hospital. There is also a strong association with GvHD but it is uncertain as to which comes first.
It is of utmost importance to reach a diagnosis of active CMV infection early. Urine, saliva, blood and bronchioalveolar lavage specimens may be used for the detection of CMV by cell culture or by a rapid diagnostic method. However, the predictive value of a positive result from different specimens vary. Blood and BAL are reported to have the highest predictive value for severe CMV disease. although they are not as sensitive as urine and saliva specimens. Saliva specimens have the added advantage of being able to yield HSV in case of active HSV infection. Many laboratories appear to have great difficulty in obtaining a positive culture or DEAFF test result from blood specimens.
Cell culture is the gold standard for diagnosing CMV infection but its value is limited by the fact that it takes several days for the characteristic CPE to appear. Rapid diagnostic methods are being increasingly used for the diagnosis of CMV infection, which include rapid culture methods such as the DEAFF test, detection of CMV antigen from polymorphonuclear cells (CMV antigenaemia), and the detection of CMV specific DNA by PCR from white blood cells or serum. The DEAFF test relies on the detection of CMV early antigens 24 to 48 hours following inoculation of the clinical specimen onto cell culture. The CMV antigenaemia test may yield a result several hours within the collection of the specimen but the test is technically tricky to carry out. PCR has been reported to be a valuable method for the diagnosis of CMV viraemia, however its sensitivity must be adjusted so that a positive result can only be obtained from those patients with active CMV infection and not from seropositive individuals with latent CMV infection. Alternatively, real-time quantitative PCR may be used but there is no standardized protocol at the moment.The current opinion is that action should be immediately taken on a positive DEAFF test or CMV antigenaemia result from the blood in non-symptomatic individuals. However, two consecutive positive results should be obtained from PCR before action is taken. It is important to standardize the total DNA used in the PCR since BMT patients typically have a very low concentration of white cells. The use of real-time quantitative PCR has proven to be of great use in the management of bone marrow transplant recipients. However, the lack of standardization of real-time PCR protocols hindered the comparison of data between centers.
Several protocols have been described for the monitoring of active CMV infection in BMT recipients. Surveillance urine and/or saliva and/or blood specimens are taken either weekly or twice weekly. BALs may be performed routinely in all recipients 1 month after transplant or in the presence of clinical symptoms. There are four main strategies for the use of antiviral agents against CMV in BMT recipients;-
1. Prophylaxis - treatment is given before and for a certain period after transplant
2. Suppression - treatment is given if CMV excretion is found at any site
3. Pre-emptive treatment - treatment is given if CMV were isolated from BAL or blood
4. Treatment of established disease.
CMV hyperimmune globulin, acyclovir and ganciclovir had been used for prophylaxis against CMV in BMT recipients with varying degrees of success. The current opinion is that acyclovir is of some value although this has been questioned recently. Although ganciclovir prophylaxis had been shown to reduce CMV infection, it is probably too toxic for use in the routine prophylaxis of bone marrow transplantation, except perhaps for matched unrelated donor (MUD) transplants. Suppression using ganciclovir had been described for one clinical trial and was reported to have a significant effect on the reduction of CMV disease. However, current opinion is not in favor of giving treatment with ganciclovir if CMV excretion is detected from the urine or saliva. Instead, consideration should be given to the relaxation of immunosuppression. Preemptive treatment with ganciclovir is now routinely given in many centres if CMV is detected from BAL or blood. Clinical trials have shown that ganciclovir reduced the development of CMV pneumonitis and death. Once CMV pneumonitis or other CMV disease is established, treatment is difficult. CMV hyperimmune globulin in conjunction with ganciclovir had been shown to have some benefit in established cases of CMV pneumonitis.
It is recognized that different types of BMT carry different
levels of risk for severe CMV disease. Patients undergoing
autologus transplants are at the lowest risk. Patients receiving
allogeneic transplants from matched sibling are at a much
increased risk, while those who have received marrow from a
matched unrelated donor (MUD) are at the highest risk (since
minor histocompatibility antigens are extremely unlikely to be
matched in this case). Therefore, some BMT units are giving
ganciclovir prophylaxis for all MUD transplants.
CMV_Infection_in_Solid Organ Transplant Recipients
Renal Transplants
CMV infection is ubiquitous in renal transplant patients, occurring in up to 80% of all patients and is the most common pathogen. Most of these infections are due to reactivation and are asymptomatic. Primary infection occurs less commonly but account for the majority of clinical CMV disease. Clinical CMV disease may comprise of fever, leukopenia, pneumonitis, retinitis, enteritis, hepatitis and encephalitis. CMV infection also leads to acute deterioration in graft function and it may also result in immunosuppression and thus allowing infection by other opportunistic agents. Seronegative patients who receive transplants from seropositive donors are at the greatest risk of developing CMV disease. Patients who are seropositive for CMV are also at risk, with 20 to 30% developing clinical illness, infection resulting either from reactivation or reinfection with another strain of CMV. There is no evidence to suggest that CMV directly causes allograft rejection or glomerulonephritis although patients with CMV disease have higher rates of allograft loss. As in the case of bone marrow transplants, viraemia is the best prognostic indicator of clinical CMV disease.
Interferon, high dose acyclovir, CMV-specific immunoglobulin
for seronegative patients given grafts from seropositive donors
had been reported as effective prophylaxis measures in reducing
clinical CMV disease. Donor-recipient CMV antibody status
matching is desirable. Although the efficacy of high dose
acyclovir and hyperimmune globulin had been questioned recently.
The use of a -interferon had been
associated with frequent irreversible rejection reactions and
should not be used. Pre-emptive treatment with ganciclovir should
be considered for those at high risk of progression to
symptomatic CMV disease such as those with viraemia or
seropositive recipients receiving antilymphocyte serum.
Heart_transplants
Primary and recurrent CMV infection occurs in heart and heart-
lung transplant recipients. Most fatal CMV infections arise in
seronegative recipients of hearts from seropositive donors. CMV
infection is more severe when acquired from the donor organ than
blood or blood products. Those CMV antibody-positive recipients
who received organs from seropositive donors were reported to
have more severe recurrent CMV disease than those who received
organs from CMV seronegative donors. The severity of CMV disease
is dependent on the degree of immunosuppression and heart-lung
transplant recipients had more serious CMV disease than heart
transplant patients. CMV donor-recipient matching is carried out
if possible. The use of CMV hyperimmune globulin as a prophylaxis
was found to have a beneficial effect, although primary infection
was not prevented, the severity of infection was reduced.
Ganciclovir had also been used as a prophylactic agent in a
recent clinical trial and was reported to have significant
clinical benefit. The role of acyclovir is uncertain. Pre-emptive
treatment with ganciclovir should be considered for those at high
risk of progression to symptomatic CMV disease such as those with
viraemia.
Liver_transplant_recipients
CMV infection is a major cause of morbidity and mortality
following liver transplantation. Both primary and recurrent
infection are common. Risk factors include donor seropositivity,
the use of antilymphocyte preparations, and re-transplantation.
Liver is the most involved organ, followed by the lung, the GI
tract, and the retina. Donor-recipient CMV antibody status
matching is recommended. CMV hyperimmune globulin had been
reported as an effective form of prophylaxis, although it had
been reported to have no effect on the highest risk group, namely
the R-D+ group. Acyclovir had been tried as prophylaxis and had
been reported to have little value. Ganciclovir or valganciclovir prophylaxis may
be of value.
Post-transplant surveillance
The current feeling is that routine
post-transplant surveillance is not necessary for most solid
organ transplant recipients. This is because even though CMV
infection is just as common as in BMT patients, the disease
manifestation rate is much lower, and even if disease is present,
it is much more manageable than in BMT patients. However, routine
surveillance should be considered for those at particularly high
risk eg. on high dose steroids or other immunosuppression.