Astroviruses of Diarrhoeal Viruses Infection
The structure of astroviruses is unique amongst human viruses and
they have been found so far only in the gut. They are so called
because of their surface has a star-shaped configuration, which
is not seen on all particles. Astroviruses are spherical
particles with a mean diameter of 28 nm. Its structure is unknown
but is unlikely to be based on an icosahedron, with which the 6
pointed star is incompatible. Animal astroviruses have a positive
stranded RNA. 5 serotypes of human astroviruses have now been
described by neutralization tests. There is no group antigen.
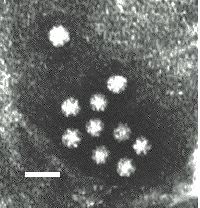
Electronmicrograph of astrovirus particles with the five pointed star structure clearly visible on the surface.
Association with disease
Astroviruses, like rota and adenoviruses may be found in the stool in large numbers. They may also be found in the stools of normal babies but more frequently in the stools of those suffering from diarrhoea. They are often hard to recognize under electron microscopy and thus they are probably considerably under- reported. Volunteer studies demonstrated that adults can be infected with the development of diarrhoeal symptoms in some cases. In addition, the majority seroconverted. However, this does not mean they have a significant role in causing diarrhoeal diseases in babies and young children. Overall it is still uncertain whether and to what extent astroviruses play a role in the causation of diarrhoeal diseases although on balance, the evidence tilts towards a causative role.
Immunity and its transfer
Antibody is induced following infection but it is uncertain whether it confers protection. A survey carried out in the UK showed that by the age of 3, the majority of children have acquired antibody against astroviruses. This pattern of antibody acquisition is similar to rotaviruses, suggesting that infection is common and that many such infections are unrecognized.
Norwalk_Virus_and_Small_Round_Structured_Viruses
This virus was the first fastidious enteric virus to be discovered, following an outbreak affecting both children and adults in a primary school. In subsequent years a number of outbreaks in summer camps, cruise ships and other gatherings have been associated with this virus. Antibodies against this virus are acquired later in life, indicating a less widespread distribution, in the USA at least. At the age of 50, only 50% of the population surveyed had antibodies. In a similar study in Bangladesh, antibodies were acquired earlier and the pattern was closer to that of rotaviruses in that the majority of the population had acquired antibody by the age of 3. Morphologically similar viruses have been found in Europe and elsewhere but their relationship to the Norwalk agent is uncertain.
Norwalk virus was originally described as parvovirus-like and 28nm in diameter. On electron microscopy, its surface has a ragged structured appearance. this was originally thought to be antibody against the virus binding to the surface as the first electron micrographs were obtained by immune electron microscopy. However, it is mow thought that the ragged outer edge is part of the surface of the virus particle. If so, the true mean diameter of the Norwalk virus particle is at least 33nm. The virus is thought to have a genome consisting of positive stranded ssRNA. Norwalk virus has not been shown to grow in cell culture with or without the addition of trypsin. Even partial growth has not been described. When present in the stool, the virus is difficult to detect by electron microscopy as the concentration of the virus in stool culture is much lower than that of rota and adenoviruses. The difficulties of finding Norwalk by electron microscopy have resulted in most infections being diagnosed by antibody tests. however, such tests will only diagnose infections by Norwalk virus itself and other similar viruses if they share a common group antigen. There is some cross-reactivity between Norwalk and Montgomery County viruses but the Hawaii agent appears to be antigenically distinct.
There is substantial evidence to suggest that SRSVs may be
members of caliciviridae. They have similar sizes and buoyant
densities, and have a major protein of very similar molecular
weight to that of caliciviruses. There is increasing serological
evidence of cross-reaction between strains of calicivirus and
SRSV. At present, at least 3 serotypes of Norwalk-like viruses
have been described from the US, 4 from the UK and at least 6
from Japan. Recently, nucleotide sequences for several NLVs have
become available which should greatly facilitate research on
these viruses. On the basis of genetic analysis, there appears to
be two main genotypes of Norwalk-like viruses, each with a large
number of subtypes. With a dramtic increase in sensitivity
compared to EM, PCR assays are now being increasingly used
for the diagnosis and investigatrion of outbreaks caused by
Norwalk-like viruses.
Electronmicrograph of Norwalk-like virus particles. Note that unlike astroviruses, it is extremely rare to find such as large number of virus particles congregated together like this.
Association with Disease
A small series of volunteer experiments confirmed that the virus was capable of causing disease in adults. Half the volunteers challenged developed illness although none were severely ill. These volunteers were rechallenged between 27 and 41 months later and those who were ill on the first occasion were ill again. Virus was detected in the faeces of those challenged. The volunteers developed both serum and secretory antibodies in the gut and it is surprising that this appeared to confer no protection. Norwalk virus has been associated almost exclusively with outbreaks of diarrhoea and vomiting. Vomiting appears to be a more prominent feature of infection than with rota and adenoviruses. Outbreaks have been associated with contaminated seafood such as oysters. Person to person spread is probably a component of almost all outbreaks involving SRSVs. Outbreaks in institutions have an epidemic curve typical of person to person spread. Faecal oral spread is probably the major means of transmission. However other routes are possible such as through environmental contamination through vomiting and possibly through the airbourne route. Outbreaks of SRSV have also been associated with exposure to recreational water such as swimming in contaminated lakes. Occasionally SRSVs are responsible for sporadic cases of gastroenteritis.
Other_Possible_Enteric_Viruses
A. Caliciviruses
Caliciviruses have been known to infect pigs, cats, sea-lions and fur-seals but it was not until 1976 that a calicivirus was fond in human faeces. The human calicivirus is morphologically indistinguishable from these animal strains. However, human calicivirus strains are antigenically distinct from animal strains. Human caliciviruses contain a single positive stranded RNA genome. and are about 33nm in diameter. The virus shows icosahedral symmetry, the face and the apices of the icosahedron are represented by cupped hollows, of which there are 32. This gives a virus a very characteristic appearance. However, not all the particles in the stool show the characteristic hollows and these particles can resemble Norwalk agents and SRSVs very closely. 4 serotypes of human calicivirus have been recognized, 3 in the UK, and 1 in Japan. No group antigen has been detected.
Electromicrograph of classical calicivirus particles. Note the charcteristic cupped shaped depressions on the surface
3 of the 4 human serotypes described are associated with
outbreaks of diarrhoea and vomiting. Vomiting being a more
prominent feature than diarrhoea and in this way and the epidemic
nature of the viruses, infection by human caliciviruses resemble
that by Norwalk agents. Caliciviruses have also been reported
infrequently in the stools of normal babies. However it is not an
easy virus to find and considerable under reporting may have
taken place. Antibody against caliciviruses may be no more
protective than with Norwalk, a further point of similarity with
Norwalk agents. Volunteer studies in the UK and USA have shown
the viruses to be capable of inducing disease in adult
volunteers. The incubation period being 24 - 72 hours with
symptoms of N+V, abdo pain and diarrhoea. Virus is excreted in
the faeces during the illness. A serological survey carried out
in Japan showed that the majority had acquired antibody in the
first three years of life. The pattern of acquisition of antibody
closely parallels the acquisition of antibody to rotaviruses and
astroviruses, suggesting this virus is widespread although few
actual infections are diagnosed.
B. Small Round Viruses
Small round virus-like objects are often seen in human faeces.
SRVs show consideration variation in size. Some of the smaller
SRVs have a diameter of 22nm and are often hexagonal in outline
so that they resemble parvoviruses. In some instances,
adenoviruses may also be found in the same stool and thus these
are probably defective adenovirus-associated virus. However,
adenoviruses are not always detectable and it may well be in
those instances that one is dealing with an autonomous
parvovirus. Indeed, certain animal parvoviruses e.g. canine
parvovirus, can cause severe diarrhoeal disease in animals. SRVs
in the next larger size band are about 25nm in diameter and
resemble enteroviruses. It is uncertain whether these are new
strains of enteroviruses but these viruses do not grow in cell
culture. Larger SRVs may occasionally be seen but it is doubtful
whether these are viruses. Occasionally, cubic bacteriophages may
be misdiagnosed as SRVs. SRVs may be present in a considerable
number of stools of infants. However, when detectable, the
numbers are relatively small. They are more likely to be found in
common source outbreaks. Both SRVs and SRSVs have been associated
with common source outbreaks, of which a considerable proportion
is associated with the consumption of shellfish, particularly
oysters. The incubation period is 36-48 hours, following which
the patient develops vomiting and diarrhoea. At present, there
are no known animal parallels.
C. Coronaviruses
Coronavirus-like particles are occasionally seen in stool extracts. The particles seen in the stools differ from the human respiratory coronaviruses in morphology. The particles in the stools have projections that resemble pins with a narrow shaft whereas those seen in the respiratory tract have club-shaped surface projections. The identities of these particles as true viruses have yet to be proved. Most attempts to culture coronaviruses have failed although there was a single unconfirmed report of growth in fetal intestinal cells. These particles have been seen in the faeces of adults as well as children. Prolonged excretion following recovery is common. Most reports associate these viruses with endemic rather than epidemic diarrhoea. No volunteer experiments have been reported to date. Coronaviruses are well established as causes of diarrhoea in animals, particularly in swine.
D. Other_Virus-like_Particles_Seen_in_Faeces
Breda-like Agents ;- Virus-like particles resembling those previously described in the faeces in cows have been seen in the faeces of a few children. Although an association with diarrhoea in man has been reported, further information is needed.
Bacteriophages ;- The human gut contains vast numbers of bacteria which are potential hosts for bacteriophages. Like animal viruses, bacteriophages come in a variety of sizes and shapes. However, the tailed bacteriophage has a structure unique to this class of virus. It is possible for cubic bacteriophages to be mistaken for SRVs. Moreover, a possible role for these phages in the causation of human diarrhoea should not be totally overlooked.