Poliomyelitis of Enteroviruses Infection
Poliomyelitis
There are 3 serotypes of poliovirus with no common poliovirus antigen. They have identical physical properties and their base sequences share 36 - 52% homology. Humans are the only natural hosts for polioviruses, Old World monkeys and chimpanzees are susceptible to infection. Antigenic variants of types 1 and 2 have been reported, however these antigenic differences do not affect the capacity of Abs induced by one strain to protect against other strains of the same type. Despite these minor intratypic differences, polioviruses show marked antigenic stability. It is important to note that other enteroviruses are occasionally associated with a polio-like illness, in particular Coxsackievirus A7 and enterovirus 71.
A. Epidemiology
Polioviruses are disseminated globally. In densely populated developing countries, almost 100% of the population have Abs to all 3 types of the virus before 5 years of age. Epidemics do not occur and paralytic disease is rare as the incidence of paralytic poliomyelitis increases with age, especially after 15 years of age. In countries with improved sanitation, infection is often delayed until adulthood with a consequent increase in the number of cases of paralytic poliomyelitis. With the advent of immunization, poliomyelitis is on the verge of eradication in many countries. Poliomyelitis occurs primarily in the summer, like the common diarrhoeal diseases. The patient is maximally contagious during the first week of illness, when the virus is excreted both in the pharynx and faeces, but the virus continues to be excreted in the faeces for to 5 to 17 weeks after the onset of illness.
B. Clinical Features
The incubation period is usually 7 - 14 days (range 3 - 35 days). Following ingestion, the virus multiplies in the oropharyngeal and intestinal mucosa. The lymphatic system, in particular the tonsils and the Peyer's patches of the ileum are invaded and the virus enters the blood resulting in a transient viraemia. In a minority of cases, the virus may involve the CNS following dissemination. The following are the possible outcomes following poliovirus infection: -
C. Laboratory Diagnosis
1. Virus isolation - the CSF usually show the changes typical for that of viral meningitis with lymphocytosis and a high protein level. However, poliovirus is rarely recovered from the CSF, in contrast to coxsackie and echoviruses. Poliovirus is readily isolated from throat swabs, faeces, or rectal swabs by inoculation into cell culture. The CPE produced can be neutralized by type specific sera which forms the basis for identification. The isolate may be further typed by molecular assays.
2. Serology - this is not widely used as virus tissue culture techniques are so efficient. Neutralization tests, in which acute and convalescent sera are mixed with known concentrations of laboratory strains of poliovirus and then absorbed onto monolayers of cell cultures, are the most efficient serological test system available. Alternatively, CFTs can be used but these are much less reliable.
D. Prevention
No specific treatment is available except supportive measures in paralytic poliomyelitis. However, it is possible to prevent the disease through active immunization. Three major discoveries were responsible for the development of successful vaccines: (1) Protection is required against all 3 types of poliovirus, (2) Poliovirus will replicate readily in cell cultures derived from non nervous tissue, and (3) viraemia is essential for the pathogenesis of paralytic poliomyelitis so that serum antibodies should interrupt the viraemia. There are 2 vaccines available: (1) the inactivated Salk vaccine, and (2) the attenuated Sabin vaccine.
1. Inactivated Salk Vaccine - the formalin inactivated intramuscular polio vaccine (IPV) is now of high potency and purity. It is both safe and effective. The use of IPV in Finland and Sweden has virtually eliminated paralytic poliomyelitis in these countries. IPV does not induce local IgA mediated immunity to polioviruses in the gut. However, IPV had been shown to confer herd immunity against poliovirus. IPV does reduce pharyngeal, and to a lesser extent, faecal shedding of the virus in vaccinated individuals who have been infected by poliovirus in the gut. This may explain the apparent success of IPV in dramatically reducing the circulation of polioviruses in countries that use IPV exclusively. It is postulated that in these countries where the standard of hygiene is very high, pharyngeal shedding is probably the major source of infection.
Recently a small outbreak of poliomyelitis where a type 3 strain was involved was reported in Finland, where IPV had been exclusively used and poliomyelitis had been unknown for 20 years. However the type 3 strain isolated was shown to have differences in antigenicity compared to the classical type 3 strains. Also, the IPV used in Finland was of less than optimal potency. IPV does confer herd immunity; whether this is by interrupting pharyngeal excretion or by reducing stool virus excretion is uncertain.
2. Live Attenuated Vaccine - the live attenuated oral polio vaccine (OPV) has several advantages over IPV: (1) it induces long lasting immunity, similar to that seen after natural infection, (2) induces IgA formation and thus local immunity against reinfection in the pharynx and gut, and (3) inexpensive mass immunization without the need for expensive sterile equipment. OPV induces a secretory IgA response that is not seen with IPV. This local mucosal response is regarded as many as the crucial argument in favour of OPV. However, mucosal immunity is not life-long and reinfection is possible within a few months although excretion is short-lived. An advantage of OPV has always seemed to be greater herd immunity. This alleged benefit is based on 3 factors; (1) stimulation of mucosal immunity and resultant curtailment of spread of wild virus (2) displacement of wild virus in the community by vaccine related strains.
When properly administered, OPV is extremely effective, as shown by the dramatic decrease in poliomyelitis since its introduction in Europe and N. America. However, the vaccine strains, in particular the type 3 strains, can revert to virulence and cause disease in those who have just been vaccinated. It is estimated that vaccine induced poliomyelitis is seen at a rate of 1 in 3,000,000 vaccinations. The actual frequency is greater in immunocompromised children and adult males. The majority of cases of paralytic polio seen in many developed countries which uses OPV are associated with the vaccine rather than wild type virus. Although the response rate is close to 100% in developed countries, the response rate is poor in some developing countries with response rates ranging from 50 - 90% for each strain. In contrast to IPV which maintains response rates close to 100% in such countries. It is not entirely clear why this should be so. An important factor is the loss of potency of the vaccine due to failure in refrigeration. Other possible mechanisms include the interference by other enteroviruses, presence of inhibitors in the GI secretions etc.
In view of the rare chance of reversal to neurovirulence by the strains of virus used in OPV, and also the fact that the response rate to OPV may be poor in developing countries with a warm climate, there is an ongoing argument on whether a switch should be made to IPV. Certainly, IPV can be combined with the DPT vaccine. However recent work carried out on the molecular basis of attenuation of type 3 poliovirus (Sabin vaccine strain) and of the reversal of the vaccine to virulence has provided information on the specific nucleotides of the poliovirus genome which affects virulence. In particular, a single nucleotide change at position 472 in the noncoding region of the genome. All 3 vaccine strains show some tendency to revert to neurovirulence. For type 3, back mutation at nucleotide 472 usually occurs within a few days of vaccine administration. Similar changes has been observed for poliovirus type 2 and type 1. Such rapid partial reversions towards virulence have given a basis of concern about the safety of OPV. In practice, the safety record is impressive. One reason for the safety of type 3 strains, despite the reversions, is probably the frequency of intratypic recombinations with type 1 and 2 viruses. Such information may lead to the development of vaccine viruses with reduced capacities to revert to virulence. In addition, more stable OPV may become available which should improve the response rate in the tropics.
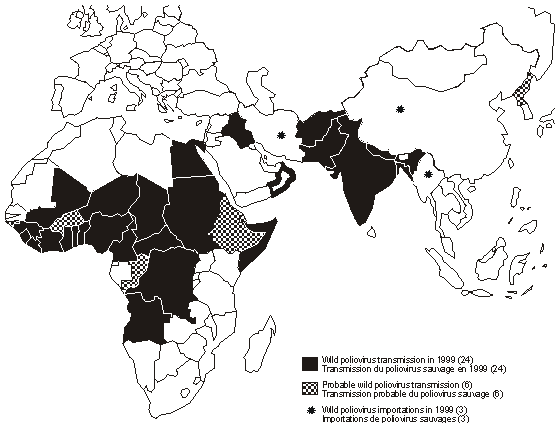
WHO Poliovirus Eradication Campaign - Poliovirus was targeted for eradication by the WHO by the end of year 2000 (now 2005). To this end, an extensive monitoring network had been set up. Poliovirus has been eradicated from most regions of the world except the Indian subcontinent and sub-Saharan Africa. It is possible that the WHO target may be achieved.

Current status of Poliovirus transmission as of 1999 (WHO).
Coxsackieviruses are distinguished from other enteroviruses by their pathogenicity for suckling rather than adult mice. They are divided into 2 groups on the basis of the lesions observed in suckling mice. Group A viruses produce a diffuse myositis with acute inflammation and necrosis of fibers of voluntary muscles. Group B viruses produce focal areas of degeneration in the brain, necrosis in the skeletal muscles, and inflammatory changes in the dorsal fat pads, the pancreas and occasionally the myocardium. Typically, group A viruses produce a flaccid paralysis whilst group B viruses produce a spastic paralysis. Each of the 23 group A and 6 group B coxsackieviruses have a type specific antigen. In addition, all from group B and one from group A (A9) share a group Ag. Cross-reactivities have also been demonstrated between several group A viruses but no common group antigen has been found. (NB. coxsackie A23 was found to be identical to echovirus 9 and the A23 has been dropped and the number is unused)
The first echoviruses were accidentally discovered in human faeces, unassociated with human disease during epidemiological studies of polioviruses. The viruses were named echoviruses (enteric, cytopathic, human, orphan viruses). These viruses were picornaviruses isolated from the GI tract, produced CPE in cell cultures, did not induce detectable pathological lesions in suckling mice. Altogether, 34 viruses were assigned echovirus serotype designations but echovirus 10 and 28 were reclassified as a reovirus and a rhinovirus respectively and the numbers are now unused. Of the 32 echoviruses, 10 show haemagglutinating activity with human group O erythrocytes, the haemagglutinin is thought to be an integral part of the virus particle. There is no group echovirus Ag but heterotypic cross-reactions occur between a few pairs. At least 14 of the known viruses produce disease in rhesus and cynomolgus monkeys if inoculated intracerebrally or intraspinally. As with polioviruses, the mouth is the portal of entry of the viruses. Although a few can probably infect through the respiratory route. They are excreted in the pharynx and faeces early in the course of infection and virus may be isolated from the faeces up to several weeks after recovery. The incubation period varies between 2 - 7 days which may be followed by one of several different disease manifestations. Recovery from infection is accompanied by the development of lifelong immunity.
A. Clincial Manifestations
The coxsackieviruses can produce a remarkable variety of diseases and even the same virus can produce different types of disease. Still, a number of group A viruses have not definitely been implicated as causative agents of any human disease. Some syndromes are almost caused exclusively by group A viruses (herpangina, hand-foot-mouth disease), some others by group B (epidemic pleurodynia, myocarditis of the newborn). The majority of syndromes can be caused by viruses of both groups.
Clinical Syndrome Group Predominant Types
1. Aseptic meningitis A 2, 4-7, 9 ,10, 12, 16
B All2. Paralytic disease A 4, 7, 9
B 3-53. Herpangina A 1-6, 8-10, 16, 21, 22
4. Hand-foot-mouth disease A 16 (rarely 4, 5, 9, and 10)
5. Fever, exanthema A 2, 4, 9, 16
B 46. Acute URTI (cold) A 2, 10, 21, 24
B 2-57. Epidemic pleurodynia A (very rarely)
B 1-58. Myocarditis of the newborn B 2-5
9. Myocarditis in children B 2-5
10.Pericarditis B 1-5
11.Undifferentiated febrile All All
illness
Other possible disease associations include diabetes and
pancreatitis and post-viral fatigue syndrome and Reye's syndrome.
Diseases associated with echoviruses
1. Aseptic meningitis
2. Encephalitis
3. Paralysis
4. Rash, fever
5. Acute upper respiratory tract infection
6. Enteritis
7. Pleurodynia
8. Myocarditis
9. Neonatal infections
Summary of clinical sydromes associated with enteroviruses
| Syndrome | Polio | Cox A | Cox B | Echo |
| Paralytic disease | + | + | + | + |
| Meningitis-encephalitis | + | + | + | + |
| Carditis | + | + | + | + |
| Neonatal disease | - | - | + | + |
| Pleurodynia | - | - | + | - |
| Herpangina | - | + | - | - |
| Rash disease | - | + | + | + |
| Respiratory infections | + | + | + | + |
| Undifferentiated fever | + | + | + | + |
| Diabetes/pancreatitis | - | - | + | - |
| Disease in immunocomp. | + | + | - | + |
A. Aseptic Meningitis - coxsackievirus meningitis is seen most frequently in children under 5 years of age. Complete recovery is the seen in almost all cases, in contrast to polioviruses. Aseptic meningitis is caused by coxsackieviruses of both groups A and B, and many echoviruses. Enterovirus 71 has been associated with meningitis and severe disease of the CNS which includes polio like paralysis.
B. Encephalitis - a generalized or focal encephalitis may be seen in association with aseptic meningitis or with minimal meningeal involvement. Children and young adults are most frequently affected. Most patients recover completely, although a few are left with neurological sequelae or damage to the hypothalamic-pituitary axis resulting in endocrine disturbance. CSF findings are similar to that found in aseptic meningitis. Patients with hypogammaglobulinaemia may develop persistent infections in which chronic meningeal irritation or encephalitis, or sometimes a dermatomyositis-like syndrome. There is a high mortality rate and i.v. immunoglobulin has not been shown to be of great value in eradicating the virus from the CSF.
C. Herpangina - herpangina is caused by group A coxsackieviruses. This illness predominantly affect children 2 - 10 years of age and is characterized by fever, sore throat and pain on swallowing, often accompanied by vomiting and abdominal symptoms. Small vesicular lesions are seen on the fauces, pharynx, palate, uvula and tonsils. Recovery is usually uneventful.
D. Hand, foot and mouth disease - this disease is usually caused by group A coxsackieviruses, although some group B viruses and enteroviruses have been reported to have caused some outbreaks. Typically, an ulcerative exanthem of the buccal mucosa is followed by painful vesicular lesions on the hands or feet. Less commonly, lesions may be present on the buttocks and genitalia. Family outbreaks are common.
E. Rubelliform rashes - a fine rubella-like maculopapular rash is often with infection by group A coxsackieviruses and echoviruses. Echovirus type 9 is most frequently implicated. Summer outbreaks, most frequently affecting children, are common. Fever, malaise and cervical lymphadenopathy may accompany the rash. Patients generally make an uneventful recovery.
F. Respiratory Infections - several enteroviruses have been associated with mild illness of the upper respiratory tract, including rhinitis (cold), particularly during the summer and autumn. Coxsackieviruses of both groups and some echoviruses have been associated. coxsackie A21 (Coe virus) has caused pharyngitis in military recruits. These viruses most commonly cause outbreaks in young children, in whom pneumonia and bronchiolitis may occur.
G. Epidemic Pleurodynia (Bornholm disease) - this is normally caused by group B coxsackieviruses, although certain group A coxsackie and echoviruses have been implicated. Outbreaks involving families are common and more extensive community wide epidemics have also been reported. The disease usually presents abruptly with fever and chest pain due to involvement of intercostal muscles. Occasionally, abdominal pain involving the abdominal muscles may simulate acute abdomen. Some patients have pain localized to the pains. Most patients recover within a week, although about 25% of patients may experiences relapses, usually within a few days of being symptom free.
H. Myopericarditis - group B coxsackieviruses are a major cause of myopericarditis. The clinical manifestations of enterovirus- induced myocarditis are variable. In neonates, the disease is usually acute and very severe with other general pathology. In adolescents and adults, it is commonly benign and may follow a subacute and chronic course and result in complications and permanent sequelae. There is now some evidence that the virus may persist after the initial infection and may lead to cardiomyopathy. Other enteroviruses, including coxsackie A and polioviruses, have been associated with myocarditis. However, non-picornaviruses can cause this disease eg. ortho and paramyxoviruses, togaviruses, herpesviruses, and adenoviruses.
The diagnosis of enterovirus-associated myocarditis is usually made on laboratory tests. Virus isolation or serological tests can be used. It is rare to isolate or detect virus antigen in heart tissue obtained as biopsy samples. In neonates, the onset is usually acute severe and often fatal. Left ventricular dilatation may be present with a pale myocardium. The endocardium and valves are normal. Viral myocarditis in adolescents and adults usually has a delayed onset and is rarely fatal. After coxsackie B viral infection, initial symptoms of URTI or GI illness is followed in 7 - 10 days with a clinical picture of pericarditis and/or progressive heart failure. Some patients do not have cardiac symptoms but have ECG changes accompanying fever, headache, myalgia. The most common symptom in acute myocarditis is chest pain, although myocarditis without cardiac involvement can be painless. Tachycardia, arrhythmias, murmurs, rubs and cardiomegaly and heart failure may be present and rarely death may result. Occasionally, systemic manifestation of disease may be noted in adults, including meningitis, hepatitis, lymphadenopathy, and splenomegaly.
I. Neonatal Infection - some coxsackie B viruses and echoviruses may cause severe and often fatal infection in newborn infants. Although there have been occasional reports of intrauterine death resulting from maternal enterovirus infection, there has been no confirmed association with congenital abnormalities. Infection may be transmitted transplacentally in late pregnancy, with the infant developing heart failure following delivery from a severe myocarditis or a meningoencephalitis. More frequently, infection is transmitted during the birth process or in postnatally via the mother or other virus-infected infants in the hospital. Some infected neonates may be asymptomatic, but others may develop illness at 3 - 7 days of age which may range from a mild febrile illness to a severe fulminating multisystem disease and death. Myocarditis, pneumonia, meningoencephalitis may occur as well as a severe hepatitis and jaundice, leading to profuse haemorrhage. Coxsackie B virus can be recovered from the faeces, brain, spinal cord and myocardium. It is important to reach a early diagnosis so that other infants can be protected. In outbreaks of neonatal infection with echo 11 virus, there is evidence to suggest that administration of HNIG containing high levels of antibodies to that virus may prevent or attenuate the infection in susceptible patients.
J. Conjunctivitis - several types of enterovirus are associated with conjunctivitis. coxsackie A24 B2, Echo 7 and 11, and enterovirus 70 have been isolated from the conjunctiva in sporadic cases. Since the early 1970s, major epidemics of acute haemorrhagic conjunctivitis have been described in Africa, the Americas and the Far East. The majority of the epidemics are due to enterovirus 70. The disease is generally localized to the eye and there is characteristic subconjunctival haemorrhage, either petechial or larger "blotches", and transient keratitis may occur. Enterovirus 70 is usually difficult to isolate and serological investigation is required. Neurological complications may occur in a few cases where a polio-like paralytic illness may occur. This occurs in 1 in 10,000 patients. The neurological involvement may develop 2 or more weeks after the onset of conjunctivitis.
K. Diabetes and pancreatitis - coxsackie B viruses are known to cause pancreatitis and diabetes in mice. There is some evidence that coxsackie B viruses, particularly B4, may play a role in the pathogenesis of juvenile onset IDDM. Postmortem studies on patients with diabetic ketoacidosis and seroepidemiological data have implicated coxsackie B viruses. 30% of children with IDDM have IgM antibodies to coxsackie B viruses compared to 5 - 8% for matched controls. It is probable that other cofactors are required.
L. Postviral Fatigue Syndrome - also known as myalgic encehalomyelitis (ME), it occurs as both sporadic and epidemic cases. It is a poorly characterized illness, the cardinal feature being excess fatiguability of the skeletal muscles. Other symptoms that may be present include muscle pain, headache, inability to concentrate, paraesthesiae, impairment of short term memory and poor visual accommodation. Focal neurological signs are rare. Evidence of myopericarditis may be present occasionally. There may be a history of a nonspecific viral illness and some lymphadenopathy may be present. Routine laboratory investigations are usually normal. Recovery usually takes place within a few weeks or months but the illness may persists in some patients with periods of remission and relapse.
The aetiology is uncertain but it is thought that there is a
substantial functional component as well as a viral component in
many cases. ME occasionally follows confirmed virus infections
such as varicella/zoster, influenza A and IM. It may follow some
bacterial infections such as toxoplasma gondii and leptospira. In
the majority of cases though, the initiating infection cannot be
diagnosed specifically. There is now substantial evidence for a
persistent enterovirus infection, particularly coxsackie B
viruses in many cases of ME. Patients with ME appears to have a
higher prevalence of antibodies against coxsackie B viruses than
matched controls. Furthermore, coxsackie B viruses may
occasionally be isolated from the faeces as well as skeletal
muscle biopsies in patients with ME.
B. Laboratory Diagnosis
1. Virus Isolation - the best specimens for isolation are faecal samples or failing that rectal swabs. Virus excretion is often intermittent and more than one specimen should be collected with an interval of 24 - 48 hours. Faecal excretion of virus commences within a few days of infection and may continue for weeks, especially with polio and coxsackie viruses but rarely exceed one month with echoviruses. Isolation is also possible from the pharynx during the acute phase of the illness, especially in cases with respiratory symptoms. (5 days before to 5 days after the onset of symptoms). The CSF should be cultured in cases of aseptic meningitis. Polioviruses are much less likely to be isolated than coxsackie and echoviruses in such cases. The isolation of enteroviruses from a normally sterile bodily fluid is much more significant than from faeces or pharyngeal secretions. The excretion of enteroviruses is common in healthy children, particularly after immunization with live polio vaccine. Occasionally, virus may also be isolated from vesicle fluids, urine and conjunctival fluids.
Primary monkey kidney and human embryo lung fibroblasts are most commonly used for the isolation of enteroviruses. All enteroviruses produce a similar CPE. Virus neutralization tests should be carried out to identify the type of virus isolated. Some enteroviruses, in particular coxsackie A, are not readily detected in cell cultures. If coxsackie A is suspected, specimens should be inoculated intracerebrally, intraperitoneally and subcutaneously into 2 or more litters of suckling mice. Group A and group B coxsackieviruses can be distinguished from each other by the pathology they induce in suckling mice. The RD cell line, derived from a human rhabdomyosarcoma, has been shown to support replication of a number of coxsackievirus group A strains (however, most strains require a passage before a CPE is apparent). The identity of the enterovirus is normally established by neutralization with type-specific antisera. However, molecular methods are increasingly used for identification.
Source: Virology Laboratory at Yale-New Haven Hospital
2. Serological Techniques - Neutralization tests are generally the best serological tests available. However they are labour intensive and takes at least 3 days before the results are available. Antibody titres are compared in paired sera, the first collected within 5 days of onset of symptoms and the second some days later. A significant rise in titre is evidence for recent infection. Significant rise in antibody titres are rare in cardiac disease as cardiac disease is usually a late consequence of coxsackie B infection.
More recently, M-antibody capture assays have become available for various coxsackie A and B, and echovirus serotypes. However, cross-reactivity between the IgM responses to different enteroviruses, including hepatitis A virus occurs. The older the patient, the more likely such heterotypic responses will occur. Enterovirus IgM usually lasts 8 - 12 weeks but may persists longer in some patients, up to a few years. It has been suggested that such a prolonged response may indicate a persistent infection in cases of recurrent pericarditis. Approximately 30 - 40% of patients with myocarditis, 60 - 70% of patients with aseptic meningitis, and 30% of patients with postviral fatigue syndrome give positive results for coxsackie B IgM. However, 10% of normal adults will also give a positive result, perhaps having experienced a recent enterovirus infection.
3. Direct detection of viral genomes - PCR
assays are becoming increasingly used for the detection and
identification of enteroviruses. They are particularly useful in
cases of suspected enterovirus meningitis where CSF is used.
C. Prevention
Vaccination is not available against coxsackie or echoviruses.
The multiplicity of antigenic types and the usually mild
manifestation of disease make the production of vaccine
impractical. The only effective measures for their control are
high standards of personal and community hygiene. Quarantine is
not effective because of the high frequency of inapparent
infections.
Newly identified picornaviruses that are not polioviruses are no longer classified separated into the species coxsackie and echovirus because of the ambiguities presented by overlapping host range variations. 5 new enteroviruses have been identified (68 - 71). Enterovirus 70 is the causative agent epidemics of acute haemorrhagic conjunctivitis that swept through Africa, Asia, India and Europe from 1969 to 1974. The virus is occasionally neurovirulent. Enterovirus 71 appears to be highly pathogenic and has been associated with epidemics of a variety of acute diseases, including aseptic meningitis, encephalitis, paralytic poliomyelitis-like disease and hand-foot-mouth disease. Enterovirus 72 is the designation assigned to hepatitis A virus, which, after it could be propagated in vitro, was shown to have the physical and chemical characteristics of enteroviruses.