Epidemiology of Human Immunodeficiency Viruses Infection
Epidemiology
The earliest unambiguously identified HIV-antibody positive serum stems from Kinhasa, Zaire dating back to 1959. It is evident that HIV infection spread unrecognized in the 1960s and 1970s. Over 100,000 cases of AIDS have been reported to the WHO by June 1988. The WHO estimates of all HIV-infected persons ranges from 5 to 10 million. Reliable estimates about the incidence and prevalence of HIV infection do not exist because of the lack of representative data. It is still unclear to what extent the mode of transmission, virus dose, cofactors, and genetic predisposition influence the infection and the incubation period. It is now thought that up to 95-99% of all HIV-infected persons eventually develop AIDS.
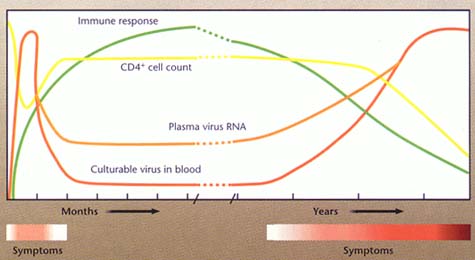
Seroconversion - the interval between infection and seroconversion was generally assumed to last about 3 to 12 weeks. Recently, serolatency of many months and up to several years have been recognized in some patients.These observations may have far- reaching implications.
Incubation period - the actual incubation period cannot be predicted in individual cases. The available evidence so far points to an median incubation period of 8 to 10 years (range: a few months to more than 14 years)
Manifestation rate - the percentage of infected persons who finally develop AIDS increases with the duration of the infection by an average of 5 to 7% per year.
Modes of transmission - HIV is transmitted by sexual contact, blood and blood products, or from the mother to the newborn infant. Other theoretical ways of transmission such as by saliva or insects do not play a role in the epidemiology of HIV infection. Evidence is increasing that the infectivity of HIV-infected individuals vary with the course of infection. It appears that infectivity is correlated with viraemia and is greater very early after infection before the appearance of antibodies and later in the course after the development of symptomatic immunodeficiency.
Risk groups - male homosexuals and bisexuals constitute the largest risk group in N. America and Western Europe. Anal intercourse and the multiplicity of partners in the case of homosexuals are the risk factors in the case of homosexuals.In contrast, heterosexual spread is the largest category in developing nations and may be associated with other sexually transmitted disease, particularly those causing genital ulcers. Prostitutes, particularly IV drug-abusing prostitutes are important multiplicators of the epidemic. The infection rate of prostitutes ranges from 0% in some European and American cities to 90% in some African cities.
HIV is transmitted by blood transfusions, clotting factors, and by contaminated needles and syringes. Blood transfusions as a cause of infection have been virtually eliminated in Western Europe and N. America since the introduction HIV antibody screening in 1985 but it continue to be a important cause of HIV transmission, particularly in Central Africa because of the high degree of infection of the donors and the lack of resources to implement the screening of the donor's blood. Infection transmitted via clotting factor have been eliminated since HIV inactivation steps have been included in their production process since 1985. IV drug abusers represent the largest AIDS patient groups in Italy (65%) and Spain (54%) and the second largest group in the US and the majority of Western European countries.
The increase in HIV-infected mothers, mostly drug-dependent is paralleled by an increase of HIV-infected newborns. The transmission rate from infected mothers to their newborn infants is about one-third. Medical personnel are not considered to be a risk group. By April 1988, only 22 cases of medical personnel infected via needle injuries and contact with the blood of HIV-infected persons have been described worldwide.
Incidence and prevalence of HIV infection - no reliable data exist on the incidence and prevalence of HIV infection in the general population. The prevalence of HIV infections in homosexuals in the US ranges from 20% to 70% in some urban centres. On the basis of studies of samples from HIV risk groups and from the general population, the total prevalence of HIV-infected persons has been estimated in several countries. The prevalence of HIV infected persons in the USA was estimated to be 1 to 1.5 million in June 1988.
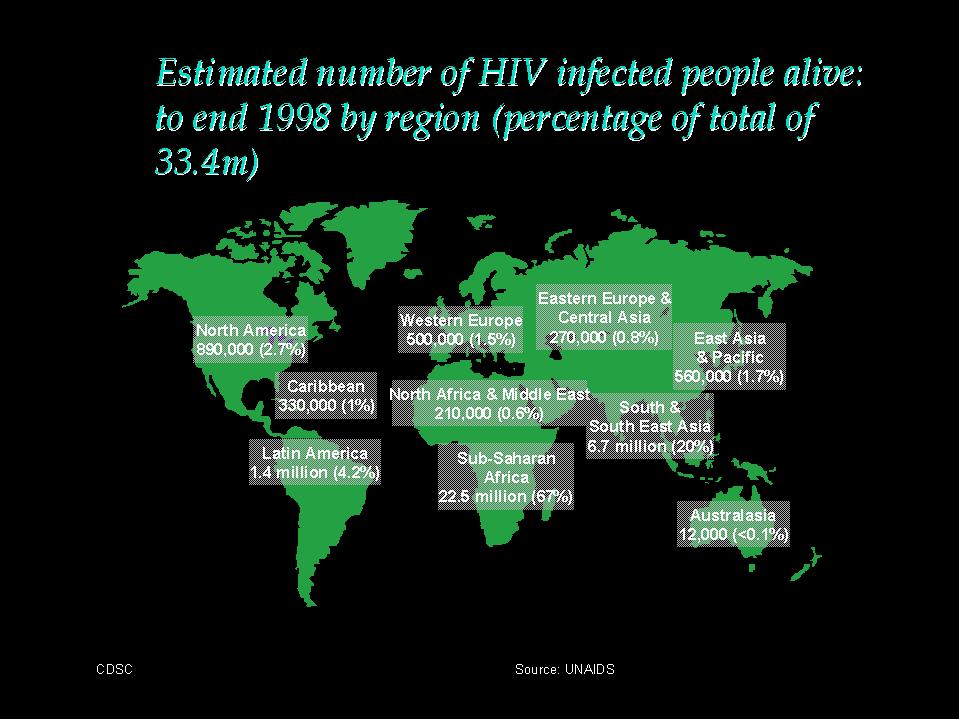
The_worldwide_distribution_of_AIDS
Africa - HIV infection is not limited to certain risk groups. The male:female ratio is 1:1 in contrast to Western Europe and N. America where the ratio is approximately 13:1. The extent of infection in the general population is considerable in some regions, mainly in the large cities and in some rural areas. In Kinshasa (Zaire) the prevalence of HIV infection was 7% in pregnant women and blood donors in early 1988. The seropositivity rate amongst blood donors in Kigali (Uganda) was 18% in 1984. The highest infection rates were found in prostitutes and in patients with sexually transmitted diseases.
Caribbean and South America - In these countries, the transmission is homosexual and heterosexual. The male:female ratio in Haiti is 4:1, suggesting that heterosexual transmission is important.
Asia and Australia - the prevalence of HIV infection in Asia is rising dramatically, especially in Thailand and India. It is predicted that HIV infection in Asia will outstrip that in Africa in due course. Most of the initial cases stem from Japan and Israel. Transmission was primarily by students from Africa and by contacts of Asian business people abroad. The epidemiology of AIDS in Australia and New Zealand is similar to that of other industrialized countries of N. America and Western Europe.
Several hypothesis regarding the origin of HIV have been developed (1) mutation of older viruses (2) transmission of animal viruses eg. SIV to man (3) transmission by an isolated remote section of the population eg. by change of sexual behavior and mobility. Available data at present favour the third possibility.

Laboratory Diagnosis
1. Serology - the diagnosis of HIV infection is usually based on serological tests.
(a) Antibody tests - ELISAs are the most frequently
used method for screening of blood samples for HIV antibody. The
sensitivity and specificity of the presently available commercial
systems approaches 100% but false positive and false negative
reactions occur. Other test systems available include passive
particle agglutination, immunofluorescence, Western blots and
RIPA bioassays. Western blots are regarded as the gold standard
and seropositivity is diagnosed when antibodies against both the
env and the gag proteins are detected. The sensitivity of the
test systems are currently being improved by the use of
recombinant antigens.
Microplate ELISA: coloured wells indicate reactivity
Interpretation of Western blot results for HIV antibody
(b) Antigen tests - HIV antigen can be detected early in the course of HIV infection before the appearance of antibody. It is undetectable during the latent period (antigen-antibody complexes are present) but become detectable during the final stages of the infection. It was argued that the routine use of antigen screening tests in the blood transfusion service may result in earlier cases of HIV infection being identified. However a large scale study carried out in the US failed to show any benefit.
2. Virus isolation - virus isolation is accomplished by the cocultivation of the patient's lymphocytes with fresh peripheral blood cells of healthy donors or with suitable culture lines such as T-lymphomas. The presence of the virus can be confirmed by reverse transcriptase assays, serological tests, or by changes in growth pattern of the indicator cells. However virus isolation is tedious and time consuming (weeks) and is successful in only 70 to 90% of cases. Therefore virus isolation is mainly used for the characterization of the virus.
3. Demonstration of viral NA - this can be accomplished by probes or by PCR techniques. The latter may be useful because of its extremely high sensitivity.
4. Prognostic Tests - the following may be useful as prognostic tests; (1) HIV antigen (2) Serial CD4 counts (3) Neopterin (4) B2-microglobulin. (5) Viral load. Of these tests, only serial CD4 counts and HIV viral load are still routinely used.
a. HIV viral load - It appears that HIV viral load has the greatest prognostic value. HIV viral load in serum may be measured by assays which detect HIV-RNA e.g. RT-PCR, NASBA, or bDNA. HIV viral load has now been established as having good prognostic value, and in monitoring response to antiviral chemotherapy. Patients with a low viral load during the incubation period had a better prognosis than those with a high viral load. Patients whose viral load decreased significantly following the commencement of antiviral therapy had a better prognosis than those who did not respond.Among patients who responded to antiviral therapy, those who had a low pre-treatment viral load had a better prognosis than those who had a high pre-treatment viral load.
b. CD4 counts - despite the increasing use of HIV-RNA
assays, measurement of CD4 still has important value in
monitoring disease progression and response to antiviral
chemotherapy. whereas CD4 count gives an indication of the stage
of disease. “The measurement of HIV viral load tells
us where the disease is going, whereas CD4 count tells us where
the disease is at this moment”
Link to IAPAC article "Monitoring Antiretroviral Therapy with Plasma HIV RNA and CD4 Counts"
5. Antiviral susceptibility assays
Because of the increasing range of anti-HIV agents available, there is increasing pressure on the provision of antiviral susceptibility assays. There are two types of antiviral susceptibility assays: phenotypic and genotypic assays
Phenotypic assays define whether a particular strain of virus is sensitive or resistant to an antiviral agent by determining the concentration of the drug needed to inhibit the growth of virus in vitro. e.g. Plaque-reduction assay for HSV, plaque-reduction assay for HIV. However, phenotypic assays can only be used for viruses that can be cultivated. Moreover, in the case of HIV, plaque reduction assays may not be that appropriate since not all HIV strains produce plaques in cell culture.
In the case of genotypic assays, mutations that are associated with resistance are assayed for by molecular biology methods such as PCR and LCR. However, these assays are tedious and are not suitable for a routine diagnostic laboratory. Moreover, he results of genotypic assays may prove very difficult to interpret since HIV mutates at a furious pace, and it is also possible that resistant strains are present right at the beginning of infection.
Link to IAPAC article "Antiretroviral
Drug Resistance and the Role of Resistance Monitoring"
Treatment
Therapy of HIV is complicated by the fact that the HIV genome is incorporated into the host cell genome and can remain there in a dormant state for prolonged periods until it is reactivated. Effective therapy must be directed against both free virus and virus-infected cells. Although a number of substances with in vitro anti-HIV activity have been described, only a few drugs exhibit anti-HIV activity in vivo at tolerable toxicities. The main group of substances described are;
1. Nucleoside analogues reverse transcriptase inhibitors. AZT, DDC, DDI and lamuvidine.
2. Non-nucleoside analogue reverse transcriptase inhibitors e.g. Nevirapine
3. HIV Protease inhibitors e.g. Ritonavir, Indivavir. They are the most potent inhibitors of HIV replication to date.
Zidovudine (AZT) was the first anti-viral agent shown to have beneficial effect against HIV infection. However, after prolonged use, AZT-resistant strains rapidly appears which limits the effect of AZT. Recent clinical trials reported significant benefit in the use of combination therapy over the use of monotherapy. The rationale for this approach is that by combining drugs that are synergistic, non-cross-resistant and no overlapping toxicity, it may be possible to reduce toxicity, improve efficacy and prevent resistance from arising. In fact, significant success has now been reported for trials involving multiple agents including protease inhibitors. The aim of anti-HIV therapy has now shifted from simply delaying the progression of disease to finding a permanent cure. We have now entered the era of highly active anti-retroviral therapy (HAART).
The current consensus is that one should give a potent combination of agents HAART right from the start when treatment is indicated. The most popular combination is AZT and lamivudine plus a protease inhibitor. Lamivudine has greater anti-retroviral activity that AZT alone and is active against many AZT-resistant strains without significant increase in toxicity.. Among protease inhibitors, indinavir (IDV) is more potent than saquinavir and appears to have fewer drug interactions and short-term adverse effects than ritonavir. In currently recommended doses, AZT prophylaxis is well-tolerated with health workers; short-term toxicity associated with higher doses primarily includes GI symptoms, fatigue, and headache. In HIV-infected adults, 3TC can cause GI symptoms, and rarely pancreatitis. IDV toxicity includes GI symptoms and after prolonged use, mild hyperbilirubinaemia (10%), and kidney stones (4%).Below is a table of some of the drugs available.
It is generally agreed that treatment should be started when
CD4<500/ml or viral load >5000 to 10000 copies/ml (bDNA
assay). If CD4 count >500/ml but viral load >5000 to 10000
copies/ml (bDNA assay), then recommendations vary. It may then be
advisable to treat those who are compliant and committed.
A. Nucleoside Reverse Transcriptase Inhibitor
| 1. AZT
2. ddI 3. ddC 4. Stavudine 5. Lamivudine (3TC) 6. Abacavir |
200 mg po or
IV tds 125-250 mg bd 0.75 mg q8h 30-40 mg bd 150 mg bd 300 mg bd |
Bone marrow
suppression (anaemia, granulocytopenia), headache,
malaise, nausea Pancreatitis, peripheral neuropathy, minor GI and CNS symptoms Peripheral neuropathy, oral ulcers, minor GI and CNS symptoms Peripheral neuropathy, minor GI and CNS symptoms Well-tolerated, minor GI and CNS symptoms reported may be due to AZT rather than 3TC. Pancreatitis was reported in 15% of patients in paediatric trials 3% develop hypersensitivity reaction: fever malaise, rash, GI symptoms. |
B. Non-Nucleoside Reverse Transcriptase Inhibitor
| 1. Nevirapine
2. Delaviridine 3. Efavirenz |
200 mg po (single
dose), then 400 mg/day 400 mg qds 600mg once daily |
Rash Transient rash, P450 inhibitor
Initial dizziness, insomnia, transient rash, P450 inducer |
C. HIV Protease Inhibitors
| 1. Saquinavir
2. Indivavir 3. Ritonavir 4. Nelfrinavir 5. Amprenavir |
600 mg tds
800mg po tds 600 mg po bd 750 mg tds 1200 mg bd |
headache,
neutropenia kidney stones, bilirubinaemia Although side-effects are common, they are usually mild and consist mainly of GI symptoms Diarrhoea common, which may respond to Ultrase MT20 enzyme preparations; occasional nausea Rash (20%), diarrhoea, nausea |
Monitoring anti-HIV therapy
1. Viral Load
Initiation - viral load is now the preferred method of monitoring therapy. There should be >= 1 log reduction in viral load, preferably to less than 10,000 copies/ml HIV-RNA within 2-4 weeks after the commencement of treatment. If <0.5 log reduction in viral, or HIV-RNA stays above 100,000, then the treatment should be adjusted by either adding or switching drugs.
Monitoring - viral load measurement should be repeated every 4-6 months if patient is clinically stable. If viral load returns to 0.3-0.5 log of pre-treatment levels, then the therapy is no longer working and should be changed.
2. CD4 count
Initiation - within 2-4 weeks of starting treatment, CD4 count should be increased by at least 30 cells/mm3. If this is not achieved, then the therapy should be changed..
Monitoring - CD4 counts should be obtained every 3-6 months during periods of clinical stability, and more frequently should symptomatic disease occurs. If CD4 count drops to baseline (or below 50% of increase from pre-treatment), then the therapy should be changed
As a result of HAART, mortality from HIV has declined
continuously in the N. America and Europe. However, the long term
outlook remains uncertain. These drugs are very expensive and the
patient needs to take them for life. Therefore, there is a
question of compliance. It had been suggested that it would take
at least 7 to 10 years to eliminate all HIV particles in an
infected person.
Prevention
The risk of contracting HIV increases with the number of sexual partners. It is interesting to note that the first cases of AIDS which were reported in homosexuals admitted to having more than 100 sexual partners per year. A change in the lifestyle would obviously reduce the risk. Paediatric AIDS generally occur early in life although some children have survived congenital infection for many years. Infected infants may serve as worldwide reservoirs of AIDS if they survive infancy, as in the case of hepatitis B.
HIV-infected mothers are not recommended to have children at present and pregnancy itself would appear to be a risk factor for seropositive mothers. A recent clinical trial demonstrated the efficacy of AZT in preventing transmission of HIV from the mother to the fetus. The incidence of HIV infection in the baby was reduced by two-thirds. The regimen used in this trial included antenatal oral administration of AZT beginning at 14-34 weeks of gestation and continuing throughout pregnancy, followed by intrapartum IV AZT, and postnatal oral AZT to the infant for 6 weeks after delivery.
The spread of HIV through blood transfusion had virtually been
eliminated since the introduction of blood donor screening in
many countries. It must be borne in mind that recently infected
donors who have yet to develop antibodies will escape detection.
There had also been recent reports of new virus isolates from
patients serologically negative for HIV, yet who may have AIDS-
related symptoms. Blood products such as factor VIII now undergo
routine treatment which appears to inactivate any HIV present
effectively.
Development_of_vaccines
An effective vaccine against HIV would have to elicit protection against both free virus and virus-infected cells. HIV neutralizing antibodies. Several examples of retroviral infections eg. FeLV, suggest that neutralizing antibodies on its own may be protective. The observation that HIV-neutralizing antibodies do not prevent the progression of the disease and the induction of neutralizing antibodies in chimpanzees by HIV gp160 do not prevent infection by HIV argues against the possibility that neutralizing antibodies are sufficient for protection against infection by HIV. Individuals with high neutralizing antibody titres, however, seem to have a longer disease free interval. Cellular immune response may be important for protection and may take place through Tc cells, NK cells and ADCC mechanisms. The role of these mechanisms in the protection against HIV infection and their relevance for anti-HIV vaccination are still unclear.
Another obstacle to the development of an effective HIV vaccine may be the high genetic variability of HIV, which resides mainly in the env gene. In certain regions of the envelope gene, the variability may be as high as 50%. However, conserved regions exist in the gp120 and the gp41 region which can be recognized by HIV sera and neutralizing antibodies can be induced with peptides representing these regions. The main types of approaches to an AIDS vaccine is as follows:
Because of the serious nature of the disease and the ability of retroviruses to induce latency, it is unlikely that live attenuated or inactivated virus will ever be acceptable for human use. Live recombinant viruses such as vaccinia or adenovirus recombinant have been developed for HIV vaccination. They carry a high immunogenicity since virus replication result in large quantities of virus antigen. Both humoral and cellular immunity can be induced. A disadvantage of this approach is possible adverse effects observed after inoculation of vaccinia virus.
Synthetic peptides can be designed such that
neutralizing and T cell epitopes can be included. The problems
with synthetic peptides are related to the tertiary structures
which may be different from those of native proteins. Another
promising approach is the production of recombinant viral
antigens, especially the gene products of the env region, and the
membrane associated p17 core protein. An alternative approach
would be the use of anti-idiotype antibodies whose usefulness had
been demonstrated against several infectious agents. Passive
immunization may eventually be available for postexposure
protection. To-date, it looks like that the best hope lies in an
inactivated vaccine. Trials are being conducted with this vaccine
in Thailand.