DIAGNOSTIC METHODS IN VIROLOGY
This topic is divided into 3 sections:
A. Overview of diagnostic methods
B. More detailed information on individual methods
C. Commonly used methods for individual viruses
There is also a Powerpoint slide set to complement these notes: Virological Methods Slide Set
A. Overview of diagnostic methods
In general, diagnostic tests can be grouped into 3 categories.: (1) direct detection, (2) indirect examination (virus isolation), and (3) serology. In direct examination, the clinical specimen is examined directly for the presence of virus particles, virus antigen or viral nucleic acids. In indirect examination, the specimen into cell culture, eggs or animals in an attempt to grow the virus: this is called virus isolation. Serology actually constitute by far the bulk of the work of any virology laboratory. A serological diagnosis can be made by the detection of rising titres of antibody between acute and convalescent stages of infection, or the detection of IgM. In general, the majority of common viral infections can be diagnosed by serology. The specimen used for direction detection and virus isolation is very important. A positive result from the site of disease would be of much greater diagnostic significance than those from other sites. For example, in the case of herpes simplex encephalitis, a positive result from the CSF or the brain would be much greater significance than a positive result from an oral ulcer, since reactivation of oral herpes is common during times of stress.
1. Direct Examination of Specimen
2. Indirect Examination
3. Serology
Detection of rising titres of antibody between acute and convalescent stages of infection, or the detection of IgM in primary infection.
| Classical Techniques |
Newer Techniques |
| 1. Complement fixation tests (CFT) | 1. Radioimmunoassay (RIA) |
| 2. Haemagglutination inhibition tests | 2. Enzyme linked immunosorbent assay (EIA) |
| 3. Immunofluorescence techniques (IF) | 3. Particle agglutination |
| 4. Neutralization tests | 4. Western Blot (WB) |
| 5. Single Radial Haemolysis | 5. Recombinant immunoblot assay (RIBA), line immunoassay (Liatek) etc. |
1. Direct Examination
Direct examination methods are often also called rapid diagnostic methods because they can usually give a result either within the same or the next day. This is extremely useful in cases when the clinical management of the patient depends greatly on the rapid availability of laboratory results e.g. diagnosis of RSV infection in neonates, or severe CMV infections in immunocompromised patients. However, it is important to realize that not all direct examination methods are rapid, and conversely, virus isolation and serological methods may sometimes give a rapid result. With the advent of effective antiviral chemotherapy, rapid diagnostic methods are expected to play an increasingly important role in the diagnosis of viral infections.
1.1. Antigen Detection
Examples of antigen detection include immunofluorescence testing of nasopharyngeal aspirates for respiratory viruses e.g.. RSV, flu A, flu B, and adenoviruses, detection of rotavirus antigen in faeces, the pp65 CMV antigenaemia test, the detection of HSV and VZV in skin scrappings, and the detection of HBsAg in serum. (However, the latter is usually considered as a serological test). The main advantage of these assays is that they are rapid to perform with the result being available within a few hours. However, the technique is often tedious and time consuming, the result difficult to read and interpret, and the sensitivity and specificity poor. The quality of the specimen obtained is of utmost importance in order for the test to work properly.
(Virology Laboratory, Yale-New Haven Hospital)
1.2. Electron Microscopy (EM)
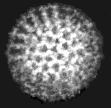
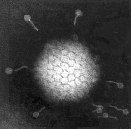
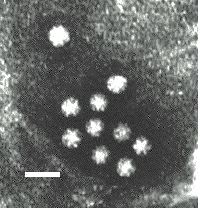
Virus particles are detected and identified on the basis of morphology. A magnification of around 50,000 is normally used. EM is now mainly used for the diagnosis of viral gastroenteritis by detecting viruses in faeces e.g. rotavirus, adenovirus, astrovirus, calicivirus and Norwalk-like viruses. Occasionally it may be used for the detection of viruses in vesicles and other skin lesions, such as herpesviruses and papillomaviruses. The sensitivity and specificity of EM may be enhanced by immune electron microscopy, whereby virus specific antibody is used to agglutinate virus particles together and thus making them easier to recognize, or to capture virus particles onto the EM grid. The main problem with EM is the expense involved in purchasing and maintaining the facility. In addition, the sensitivity of EM is often poor, with at least 105 to 106 virus particles per ml in the sample required for visualisation. Therefore the observer must be highly skilled. With the availability of reliable antigen detection and molecular methods for the detection of viruses associated with viral gastroenteritis, EM is becoming less and less widely used.
 |
 |
 |
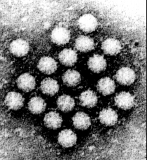 |
Electronmicrographs of viruses commonly found in stool
specimens from patients suffering from gastroenteritis. From left
to right: rotavirus, adenovirus, astroviruses, Norwalk-like
viruses. (Courtesy of Linda M. Stannard, University of Cape
Town, http://www.uct.ac.za/depts/mmi/stannard/emimages.html)
1.3. Light Microscopy
Replicating virus often produce histological changes in infected cells. These changes may be characteristic or non-specific. Viral inclusion bodies are basically collections of replicating virus particles either in the nucleus or cytoplasm. Examples of inclusion bodies include the negri bodies and cytomegalic inclusion bodies found in rabies and CMV infections respectively. Although not sensitive or specific, histology nevertheless serves as a useful adjunct in the diagnosis of certain viral infections.
1.4.Viral Genome Detection
Methods based on the detection of viral genome are also commonly known as molecular methods. It is often said that molecular methods is the future direction of viral diagnosis. However in practice, although the use of these methods is indeed increasing, the role played by molecular methods in a routine diagnostic virus laboratory is still small compared to conventional methods. It is certain though that the role of molecular methods will increase rapidly in the near future. Classical molecular techniques such as dot-blot and Southern-blot depend on the use of specific DNA/RNA probes for hybridization. The specificity of the reaction depends on the conditions used for hybridization. These techniques may allow for the quantification of DNA/RNA present in the specimen. However, it is often found that the sensitivity of these techniques is not better than conventional viral diagnostic methods.
Newer molecular techniques such as the polymerase chain
reaction (PCR), ligase chain reaction (LCR), nucleic acid based
amplification (NASBA), and branched DNA (bDNA) depend on some
form of amplification, either the target nucleic acid, or the
signal itself. bDNA is essentially a conventional hybridization
technique with increased sensitivity. However, it is not as
sensitive as PCR and other amplification techniques. PCR is the
only amplification technique which is in common use. PCR is an
extremely sensitive technique: it is possible to achieve a
sensitivity of down to 1 DNA molecule in a clinical specimen.
However, PCR has many problems, the chief among which is
contamination, since only a minute amount of contamination is
needed to give a false positive result. In addition, because PCR
is so sensitive compared to other techniques, a positive PCR
result is often very difficult to interpret as it does not
necessarily indicate the presence of disease. This problem is
particular great in the case of latent viruses such as CMV, since
latent CMV genomes may be amplified from the blood of healthy
individuals. Despite all this, PCR is being increasingly used for
the rapid diagnosis of rapid. This is especially as the cost of the assay come down
and the availability of closed automated systems that could also
perform quantification (Quantitative PCR) e.g. real-time PCR and Cobas Amplicor.systems. Other amplification
techniques such as LCR and NASBA are just as susceptible to
contamination as PCR but that is ameliorated to a great extent by
the use of propriatory closed systems. It is unlikely though that
other amplification techniques will challenge the dominance of
PCR since it is much easier to set up an house PCR assay than
other assays. One advantage of PCR assays is that the PCR product can be
sequenced and thus used for epidemiological investigation and drug
susceptibility testing.
2. Virus Isolation
Cell cultures, eggs, and animals may be used for isolation. However eggs and animals are difficult to handle and most viral diagnostic laboratories depend on cell culture only. There are 3 types of cell cultures:
2.1. Types of cell cultures
Primary cell culture are widely acknowledged as the best cell culture systems available since they support the widest range of viruses. However, they are very expensive and it is often difficult to obtain a reliable supply. Continuous cells are the most easy to handle but the range of viruses supported is often limited.
2.2. Identification of growing virus
The presence of growing virus is usually detected by:
Confirmation of the identity of the virus may be carried out using neutralization, haemadsorption- inhibition, immunofluorescence, or molecular tests.
 |
 |
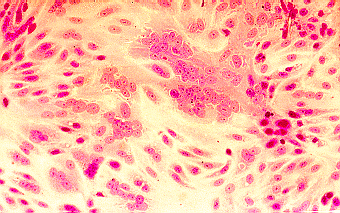 |
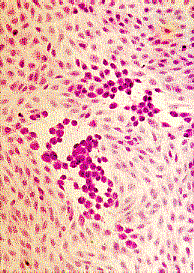
Left to Right: Cytopathic effect of HSV, enterovirus 71, and RSV in cell culture. Note the ballooning of cells in the cases of HSV and enterovirus 71. Note syncytia formation in the case of RSV. (Linda Stannard. University of Cape Town, Virology Laboratory, Yale-New Haven Hospital)
2.3 Problems with cell culture
The main problem with cell culture is the long period (up to 4 weeks) required for a result to be available. Also, the sensitivity is often poor and depends on many factors, such as the condition of the specimen, and the condition of the cell sheet. Cell cultures are also very susceptible to bacterial contamination and toxic substances in the specimen. Lastly, many viruses will not grow in cell culture at all e.g. Hepatitis B and C, Diarrhoeal viruses, parvovirus etc.
2.4 Rapid Culture Techniques
Rapid culture techniques are available whereby viral antigens
are detected 2 to 4 days after inoculation. Examples of rapid
culture techniques include shell vial cultures and the CMV DEAFF
test. In the CMV DEAFF test, the cell sheet is grown on
individual cover slips in a plastic bottle. After inoculation,
the bottle then is spun at a low speed for one hour (to speed up
the adsorption of the virus) and then incubated for 2 to 4 days.
The cover slip is then taken out and examined for the presence of
CMV early antigens by immunofluorescence.
 |
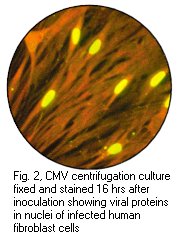 |
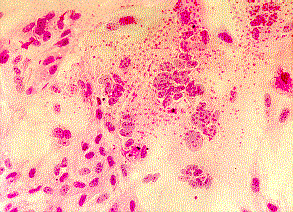
Left: Haemadsorption of red blood cells onto the surface of a cell sheet infected by mumps virus. Also note the presence of syncytia which is indistinguishable from that of RSV (Courtesy of Linda Stannard, University of Cape Town). Right: Positive CMV DEAFF test. (Virology Laboratory, Yale-New Haven Hospital)
The role of cell culture (both conventional and rapid
techniques) in the diagnosis of viral infections is being
increasingly challenged by rapid diagnostic methods i.e. antigen
detection and molecular methods. Therefore, the role of cell
culture is expected to decline in future and is likely to be
restricted to large central laboratories.
3. Serology
Serology forms the mainstay of viral diagnosis. This is what happens in a primary humoral immune response to antigen. Following exposure, the first antibody to appear is IgM, which is followed by a much higher titre of IgG. In cases of reinfection, the level of specific IgM either remain the same or rises slightly. But IgG shoots up rapidly and far more earlier than in a primary infection. Many different types of serological tests are available. With some assays such as EIA and RIA, one can look specifically for IgM or IgG, whereas with other assays such as CFT and HAI, one can only detect total antibody, which comprises mainly IgG. Some of these tests are much more sensitive than others: EIAs and radioimmunoassays are the most sensitive tests available, whereas CFT and HAI tests are not so sensitive. Newer techniques such as EIAs offer better sensitivity, specificity and reproducibility than classical techniques such as CFT and HAI. The sensitivity and specificity of the assays depend greatly on the antigen used. Assays that use recombinant protein or synthetic peptide antigens tend to be more specific than those using whole or disrupted virus particles.
3.1. Criteria for diagnosing Primary Infection
A significant rise in titre of IgG/total antibody between acute and convalescent sera - however, a significant rise is very difficult to define and depends greatly on the assay used. In the case of CFT and HAI, it is normally taken as a four-fold or greater increase in titre. The main problem is that diagnosis is usually retrospective because by the time the convalescent serum is taken, the patient had probably recovered.
Presence of IgM - EIA, RIA, and IF may be are used for the detection of IgM. This offers a rapid means of diagnosis. However, there are many problems with IgM assays, such as interference by rheumatoid factor, re-infection by the virus, and unexplained persistence of IgM years after the primary infection.
Seroconversion - this is defined as changing from a previously antibody negative state to a positive state e.g. seroconversion against HIV following a needle-stick injury, or against rubella following contact with a known case.
A single high titre of IgG (or total antibody) - this is a very unreliable means of serological diagnosis since the cut-off is very difficult to define.
3.2. Criteria for diagnosing re-infection/re-activation
It is often very difficult to differentiate re-infection/re-activation from a primary infection. Under most circumstances, it is not important to differentiate between a primary infection and re-infection. However, it is very important under certain situations, such as rubella infection in the first trimester of pregnancy: primary infection is associated with a high risk of fetal damage whereas re-infection is not. In general, a sharp large rise in antibody titres is found in re-infection whereas IgM is usually low or absent in cases of re-infection/re-activation.
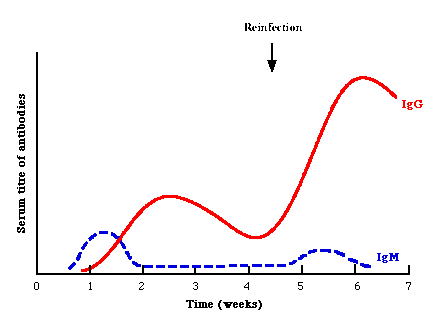
Serological events following primary infection and
reinfection. Note that in reinfection, IgM may be absent or only
present transiently at a low level.
3.3. Limitations of serological diagnosis
How useful a serological result is depends on the individual virus.
For viruses such as rubella and hepatitis A, the onset of clinical symptoms coincide with the development of antibodies. The detection of IgM or rising titres of IgG in the serum of the patient would indicate active disease.
However, many viruses often produce clinical disease before the appearance of antibodies such as respiratory and diarrhoeal viruses. So in this case, any serological diagnosis would be retrospective and therefore will not be that useful.
There are also viruses which produce clinical disease months or years after seroconversion e.g. HIV and rabies. In the case of these viruses, the mere presence of antibody is sufficient to make a definitive diagnosis.
There are a number of problems associated with serology:-
long length of time required for diagnosis for paired acute and convalescent sera
mild local infections such as HSV genitalis may not produce a detectable humoral immune response
Extensive antigenic cross-reactivity between related viruses e.g. HSV and VZV, Japanese B encephalitis and Dengue, may lead to false positive results
immunocompromised patients often give a reduced or absent humoral immune response.
Patients with infectious mononucleosis and those with connective tissue diseases such as SLE may react non-specifically giving a false positive result
Patients given blood or blood products may give a false positive result due to the transfer of antibody.
Complement Fixation Test in Microtiter Plate. Rows 1 and 2 exhibit complement fixation obtained with acute and convalescent phase serum specimens, respectively. (2-fold serum dilutions were used) The observed 4-fold increase is significant and indicates infection.
Microplate ELISA: coloured wells indicate reactivity. The darker the colour, the higher the reactivity
3.4. Antibody in the CSF
In a healthy person, there should be little or no antibodies in the CSF. Where there is a viral meningitis or encephalitis, antibodies may be produced against the virus by lymphocytes in the CSF. The finding of antibodies in the CSF is said to be significant when ratio between the titre of antibody in the serum and that in the CSF is less than 100. But this does depend on an intact blood-brain barrier. The problem is that in many cases of meningitis and encephalitis, the blood-brain barrier is damaged, so that antibodies in the serum can actually leak across into the CSF. This also happens where the lumbar puncture was traumatic in which case the spinal fluid would be bloodstained. So really, one should really check the integrity of the blood-brain barrier before making a definite diagnosis. One way to check the integrity of the blood brain barrier is to use a surrogate antibody that most individuals would have, such as measles virus, since most people would have been vaccinated. So the patient's serum and CSF for measles antibody. If the blood-brain barrier is intact, there should be little or no measles antibodies in the CSF.