Viral Haemorrhagic Fever
Viral haemorrhagic fevers are, together with AIDS and rabies among the most feared of all viral diseases. The dramatic symptoms coupled with a very high mortality rate adds much to the folklore of diseases such as Lassa fever and Ebola viruses. Fortunately these viruses are confined geographically to the African continent. The most common viral haemorrhagic fever is dengue haemorrhagic fever which is found in many parts of Asia, Africa and Latin Africa. Another viral haemorrhagic fever, which is gaining attention is hantavirus although in the case the haemorrhagic symptoms are less prominent. This section will deal with areanviruses and filoviruses. Denque and hantaviruses will be discussed in other sections of the website.
Dengue is discussed in another section under arboviruses. Another viral haemorrhagic fever, which is gaining attention is hantavirus which will be discussed in this section together with arenaviruses and filoviruses.
Arenaviruses are enveloped single-stranded RNA virus. Nearly all the members cause acute or persistent infections of rodents in Africa, Europe or America. Only certain arenaviruses cause severe haemorrhagic disease in man eg. Lassa, Machupo and Junin. The mortality of the above viral infections appear to be due to the direct cytolytic effects of these agents. In contrast, disease caused by the LCM virus appear to have an immunopathological basis.
A. Properties
Enveloped ssRNA viruses, virions 80-150nm in diameter
genome consists of 2 pieces of ambisense ssRNA
7-8 nm spikes protrude from the envelope
host cell ribosomes are usually seen inside the outer membrane and may account for 50% of total RNA content but plays no part in virus replication.
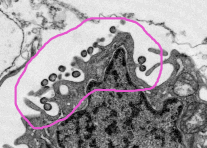
The host cell ribosomes present account for the name arena (Latin:sand). The virions bud from the infected cell's plasmalemma, beneath which the ribosomes collect.
Arenavirus particles budding from the surface of an infected cell (Source: CDC)
B. Lassa Fever
1. Epidemiology
Lassa fever is found predominantly in West Africa, extensive clinical diseases and high seroprevalence is documented in Nigeria, Sierra Leone and Liberia and the disease has also been reported from other West African countries. The actual number of cases must run into thousands or tens of thousands and the impact of the disease must be considerable. A study carried out in Sierra Leone from 1977 to 1979 reported 12% of all hospital admissions to 2 hospitals to be Lassa fever. Deaths due to Lassa fever represented 30% of all hospital deaths. Seroprevalence in villages in Sierra Leone ranged from 8% to 52%. Seroconversion to Lassa virus was found in 7% of all febrile episodes studied. Thus Lassa fever was is very common and is often the cause of mild disease in villages as well as serious disease in hospitals.
The multimammate rat (Mastomys natalensis) appears to be the natural reservoir. These rodents sustain chronic viraemia and virus shedding in the absence of histological lesions. Man to man transmission of Lassa fever have been extensively documented. Lassa fever is a well-recognized nosocomial infection e.g. in a Nigerian hospital, a single case infected 18 persons on a hospital ward of which half died. It is also apparent that the above is rare as most patients with Lassa fever do not disseminate the virus. Current recommendations are that barrier nursing procedures should be carried out with the use of surgical masks.
Mastomya natalensis (Source: CDC)
2. Clinical Presentation
The incubation period may vary from 5 days to 3 weeks after exposure. Patients typically experience the insidious onset of progressive fever, malaise, myalgia and a sore throat. Typical patchy or ulcerative pharyngeal lesions may be seen. More severe cases develop myocarditis (bradycardia), pneumonia with pleural effusion, encephalopathy (altered consciousness, convulsions and coma), haemorrhagic manifestations and shock. A mortality rate of 25% for hospitalized cases had been reported for the disease. Lassa fever carries a higher morbidity and mortality in pregnant women.
3. Laboratory Diagnosis
Virus Isolation - During the illness, the virus can be isolated from the blood, urine, pleural fluid and throat washings. Vero cells are generally used for isolation. Infected cell cultures are regularly followed by IF. It is also possible to isolate Lassa fever virus in mice but this is not practical given the high cost and the need for grade 4 containment.
Direct Detection - ELISA can be used to detect antigen in inactivated sera. The appearance of specific IgM antibodies preceded the disappearance of antigen. RT-PCR tests are also available but are mainly used in a research setting.
Serology - ELISAs for the detection of specific
IgM and IgG are now generally used for the diagnosis of
Lassa Fever. IF tests are available but have been
superseded by ELISA. Antibodies are present in the early
phases of the illness simultaneously with viral
infectivity. Using a combination of antigen and IgM
antibody tests, it was shown that virtually all Lassa
virus infections could be diagnosed early.
4. Treatment and Prevention
Good supportive care is required in the case of severe disease. Shock should be carefully managed. Any secondary bacterial infection should be sought and treated.
Ribavirin - This is a nontoxic, non-immunosuppressive nucleoside analogue with broad-spectrum antiviral activity. This drug had been shown to be effective against Lassa fever with a 2 to 3 fold decrease in mortality in high risk Lassa fever patients. Treatment should be commenced as soon as possible and should be given IV. However, the drug is embryotoxic in some experimental animals and its use is contraindicated in pregnant women.
Hyperimmune serum - the effects of hyperimmune serum is still uncertain although dramatic results have been reported in anecdotal case reports.
Postexposure prophylaxis against Lassa fever may be desirable.
There is no established safe prophylaxis against Lassa virus.
Various combinations of hyperimmune convalescent immunoglobulin
and/or oral ribavirin may be used. No effective vaccine is
available against Lassa virus at present. The development of
inactivated vaccines had been hampered by the inability to obtain
large quantities of arenavirus from cell cultures. This practical
problem, coupled with the prominent role of CMI in arenavirus
disease has directed interest towards live attenuated vaccines.
However, extreme care must be taken if and when the latter is to
be administered to humans. In the absence of effective vaccines,
the basis of prevention lies in the control of human contact with
infected rodent populations. Rodent control procedures such as
simple trapping may reduce the incidence of the disease.
C. Junin and Macupo Viruses
Junin and Macupo viruses are the causative agents of Argentine and Bolivian Haemorrhagic fever respectively. The clinical presentations are similar to that of Lassa fever, although subclinical cases are rarer. Neurological signs are much more prominent than in Lassa fever. Death may supervene due to shock secondary to blood loss or neurological complications. Unlike Lassa virus, no secondary human to human spread had been recorded. Convalescent plasma therapy had been shown to be useful and had been used since 1958. Human monoclonal antibodies may become available for use in future. However, in 11% of patients given convalescent serum, delayed neurological manifestations were seen, sometimes resulting in death. The antiviral drug ribavirin had been shown to be effective against the viruses in animal studies. The drug is well tolerated by human patients and ongoing studies may lead to it replacing antibody therapy as the treatment of choice. The rodent Calomys musculinis is the natural reservoir for the Junin virus in an endemic region in Argentina, which comprises of more than 1 million inhabitants. Infection predominantly involved adult males. No vaccine is yet available but human trials which had been carried out on a live attenuated strain with encouraging results. Good humoral and CMI had been demonstrated and no clinical reactogenicity had been observed. The rodent C callosus is the reservoir for Macupo virus in an endemic region in Bolivia encompassing a population of 50,000. The control of C callosus in and around houses has eliminated epidemic disease.
D. Lymphocytic Choriomenigitis Virus
LCM virus is the prototype member of the arenaviridae. It rarely infects humans. When it does, it is usually under conditions when the indigenously infected mouse populations is extremely dense or from contact with infected hamsters. The disease is generally mild and is manifested usually as a form of aseptic meningitis (lymphocytic meningitis) or an influenza like illness. Very rarely, severe or fatal disease is seen with haemorrhagic manifestations. LCMV may be pathogenic for the fetus. 1 woman infected during pregnancy aborted. 2 others delivered babies with hydrocephalus, chorioretinitis and mental retardation. Russian workers had reported a high prevalence of LCMV antibodies in children with hydrocephalus. On a worldwide basis, the extent of LCMV involvement in human disease is unknown as facilities for diagnosing the infection varied extensively from country to country.
In 1967, a unique viral haemorrhagic fever appeared in laboratory workers in Marburg in West Germany, which was traced to contact with the blood and tissues of a group of African Green or vervet monkeys imported from Uganda. In 1976, 2 epidemics of severe haemorrhagic fever were reported from northern Zaire and southern Sudan. The virus, which was similar to Marburg virus, was named after the Ebola river which separates Zaire and Sudan. Clinically, they cause the most severe form of viral haemorrhagic fever known, with 60 to 90% mortality.
A. Properties
Enveloped RNA virus of unique morphology, forms tubular structures of 80nm in diameter and up to 10,000nm in length.
Infectious particles are 800-900nm in length.
helical nucleocapsids surrounded by an envelope bearing 5 to 10nm projections.
Marburg and Ebola viruses are clearly different viruses in that their peptides have different molecular weights and in that they differ antigenically.
Electronmicrograph of Marburg virus particles (Source: CDC)
B. Epidemiology
Marburg virus has been identified in restricted regions of
eastern and southern Africa. Ebola virus has been isolated from
Zaire and Sudan. However, serological surveys suggests that
Marburg and Ebola viruses had been active in numerous African
countries (10 to 50% of sera showed initial reactivity, although
studies using western blots showed that the vast majority of the
sera were non- specifically reactive). What is not resolved is
why areas of high antibody prevalence do not correlate with
reported disease. Recognized disease has occurred in sporadic
cases, case clusters or epidemics. The usual pattern seen with
large outbreaks begins with a focus that disseminates infection
to a nucleus of patients, for example, to workers in a laboratory
processing African monkey tissue, or a hospital outbreak where
patients were infected by contaminated syringes. Secondary and
subsequent generations of disease occur as close members or
medical personnel are infected. It appears that the major route
of interhuman transmission requires direct contact with infective
blood or body fluids, although droplet and aerosol infections may
occur.
C. Clinical Manifestations
The onset of illness is sudden and marked by fever, chills,
headache, myalgia, and anorexia. Abdominal pain, sore throat,
N+V, cough, and arthralgia and diarrhoea are also common. A
maculopapular rash may be seen. Haemorrhagic phenomena develop at
the height of the illness, with GI bleeding being most commonly
recognized, but petechiae and mucosal haemorrhages are also seen.
Suggestive evidence of DIC is often present. The mortality is
extremely high, being in the order of 60 to 90%.
D. Diagnosis, Treatment and Prevention
Virological diagnosis is readily achieved by virus isolation
from serum during the febrile phase of the illness. Vero cells
are the most widely used laboratory system for isolation. Blood
and tissue specimens may also be inoculated into guinea pigs. In
practice, viral isolation is rarely carried out because of the
need for Class IV containment and dangers involved. Diagnosis is
usually made by the direct detection of viral antigen by ELISA or
viral-RNA by PCR, and serology. A serological diagnosis can be
made by the detection of virus- specific IgM antibodies by ELISA
and IF techniques. There is no proven virus-specific treatment.
Supportive therapy should be directed toward the maintenance of
the effective blood volume. Once cases are identified, person to
person spread must be prevented. Ordinary sterilization
techniques and barrier nursing will suffice to prevent continued
transmission. No vaccine is available and the need for one
remains to be established.
2014-2016 Outbreak in West Africa
The Ebola outbreak in West Africa was first reported in March 2014, and rapidly became the deadliest occurrence of the disease since its discovery in 1976. In fact, the epidemic killed five times more than all other known Ebola outbreaks combined, causing major loss of life and socioeconomic disruption in the region, mainly in the countries of Guinea, Liberia, and Sierra Leone. The total number of reported cases was 28637 of which 11315 people died. On 13th January 2016, the WHO declared Liberia, which was the last country to be affected, Ebola-free.
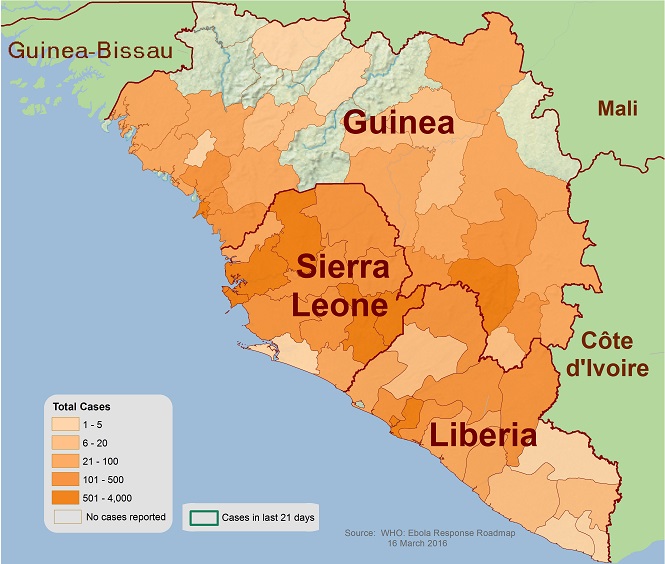
Distribution of Ebola in West Africa (2014-16)